Research Article - Biomedical Research (2017) Volume 28, Issue 6
Dynamics of hormonal profile and anti-mullerian hormone during spontaneous ovulation in PCOS women with oligomenorrhea
Fengli Zhang1,2, Jing Du2, Bin Wang2, Hongwei Wen2, Xinqiu Jia2, Huixiao Chen2 and Xiaohui Deng1*1Infertility Center, Qilu Hospital Shandong University, Jinan, Shandong Province, China
2Department of Obstetrics and Gynaecology, Liaocheng People's Hospital, Liaocheng, Shandong Province, China
- *Corresponding Author:
- Xiaohui Deng
Infertility Center
Qilu Hospital Shandong University
China
Accepted date: November 2, 2016
Abstract
Anti-Mullerian Hormone (AMH) is a glycoprotein produced by the granulosa ovarian cells with respect to the regulation of the follicular growth and development. Serum AMH levels are significantly elevated in Polycystic Ovary Syndrome (PCOS) women compared to normal controls. In 22 oligo-ovulation PCOS women and 12 anovulation PCOS women. Serum AMH, FSH, E2, LH, T, P, PRL, FINS, FBG, HOMA-IR concentrations were measured at baseline, and the hormone changes including serum E2, LH, FSH,T concentrations and follicular development were measured at the appropriate time in relation to the diameter of the follicle. The Ovary Volume (OV) (ml) and Antral Follicle Count (AFC) in anovulation group were higher compared to the oligo-ovulation group (P<0.001 and P<0.002, respectively). In the anovulation group, antral follicles had not been activated and serum T and LH concentrations maintained higher levels. AMH concentrations were constant during the growth and development of antral follicles in oligo-ovulation PCOS women, whereas they remained elevated in anovulation PCOS women during the menstrual cycle (P<0.05). In the two groups, there was a positive correlation between AMH and AFC (P=0.000 and P=0.01, respectively), as well as OV (P=0.013 and P=0.016, respectively) and T (P=0.002 and P=0.028, respectively). Constant AMH concentrations during the spontaneous initiation of follicular in oligo-ovulation PCOS women and higher serum AMH concentrations in the anovulation PCOS women suggested that AMH may be used as a marker in the diagnosis or as a predictor of ovulation in PCOS women with oligomenorrhea.
Keywords
Polycystic ovary syndrome, Oligomenorrhea, Anti-mullerian hormone, Luteinizing hormone, Testosterone, Ovulation
Introduction
Polycystic Ovary Syndrome (PCOS) is the most common endocrine disorder in women of reproductive age, affecting 6-16% of these women [1,2]. PCOS is characterized by a clustering of hyperandrogenism, hyperinsulinemia, hypersecretion of LH, menstrual disorders, and oligo/ anovulatory and infertility [3,4]. Oligoovulation occurs in 13.1% of women under 30 years old [2]. PCOS women with severe oligoamenorrhea and anovulation display a more severe phenotype characterized by more severe hyperandrogenism and a higher prevalence of abnormal metabolic and cardiovascular risk parameters than ovulatory patients with PCOS [5]. The cause of the change in ovarian function and anovulation, which affects a subgroup of these women, remains unknown.
AMH is a dimeric glycoprotein and a member of the Transforming Growth Factor-b (TGF-b) family of growth and is produced by the granulosa cells of preantral and small antral follicles in women [6], which are involved in the regulation of folliculogenesis. Because AMH is secreted exclusively in the gonads, its serum concentrations in women are thought to reflect the size of the ovarian follicle pool. Certainly, the serum AMH concentration appears to be greatly increased in most patients with PCOS [7,8]. Increased serum AMH in women with hyperandrogenism and/or oligo-anovulation could indicate to clinicians the presence of PCOS when reliable ultrasound is not available [9]. In addition, AMH levels can help to assess ovarian response potential and guide ovarian stimulation while avoiding OHSS [10]. To date, AMH has developed into a factor with a wide array of clinical applications, mainly based on its ability to represent the number of antral and pre-antral follicles present in the ovaries [11]. Saleh showed the efficacy of serum AMH measurement as a prognostic biochemical marker in the follow-up of metformin treatment of PCOS women [12]. AMH may serve as a reliable tool to characterize the severity of the syndrome, as well as for monitoring and forecasting, and it may also be used as a marker of polycystic ovaries in PCOS [2,13,14]. However, results are inconclusive regarding the effect of ovarian response on stimulation after low-dose gonadotropin in OI in patients with PCOS [15,16].
Serum AMH levels in women with PCOS are higher than in ovulatory women. However, studies devoted to the relationship between follicular development and the AMH levels in PCOS women with oligomenorrhea are limited. The aim of this study was to compare dynamic AMH concentrations, hormonal levels according to the growth and development of antral follicles in PCOS women with oligomenorrhea during menstrual cycles, providing new insights into folliculogenesis in PCOS women with oligomenorrhea and potentially offering a basis for AMH as a marker in the diagnosis or as a predictor of ovulation.
Materials and Methods
Subjects
From March 2014 to April 2015, the study consisted of 34 volunteer reproductive women between 22 and 34 years old. This study was approved by the local medical ethics review board. All participants of the study had given their written informed consent. All subjects were selected from the Outpatient Department of Gynaecology and Obstetrics in Liaocheng People’s Hospital. The diagnosis of PCOS was based on the Rotterdam criteria [17]. None of the women had any chronic diseases. In addition, none of the women had used hormones or other medications for 6 months prior to the study. Oligomenorrhea was defined as a cycle length>35 days. The women were divided into two groups: the oligo-ovulation group (n=22) and the anovulation group (n=12). Non-activated antral follicle was defined as anovulation over 60 days.
Clinical protocol
Overnight fasting blood samples were collected from all subjects in the early follicular phase (days 2-3) after spontaneous menstruation, and transvaginal ultrasounds were performed.
Age, Body Weight (BW), Body Mass Index (BMI), E2, FSH, LH, T, PRL, P concentrations, Fasting Blood Glucose (FBG), Fasting Insulin (FINS), and Homeostasis Model Assessment for Insulin Resistance (HOMA-IR) were measured on day 2-3 of spontaneous menstrual bleeding. HOMA-IR=(fasting plasma glucose (mmol/L) × fasting plasma insulin (mIU/L))/ 22.5 [18]. Then, serum E2, LH, FSH, and T concentrations and follicular development were determined every 7 days. When the follicular diameter was up to 10 mm, it was measured every 3 days until mature follicle ovulation. The final measurement was taken on seventh days (D7) after ovulation. Non-activated antral follicle was defined as anovulation over 60 days, and monitoring was stopped. Serum AMH concentration was measured on day 2-3 of the menstrual cycle (T1) at selection of the dominant follicle (T2); the time of mature follicle (T3) in oligo-ovulation PCOS women was taken once, whereas in anovulatory PCOS women, it was taken at two time points (T4=on day 2-3 of the menstrual cycle; T5=on approximately day 60 of the menstrual cycle). The day of selection of the dominant follicle was defined as the day on which a follicle reached a diameter of 10 mm or larger and remained enlarged during subsequent days until ovulation.
Transvaginal US (TVUS) was performed on each patient using a 6.5 MHz endovaginal probe. Ovarian volume was calculated for each ovary using the formula for a prolate ellipsoid: 0.5 × length × width × thickness. Ovarian Volume per ovary (OV) was defined as the average of the ovarian volume obtained from both ovaries [19].
Assay methods
Serum levels of E2, FSH, LH, T, PRL, and P concentrations were determined by electrochemiluminescence immunoassay using the E170 kit (BioMerieux, SA, France) (using an immunometric luminescence assay). FBG and FINs were measured by chemiluminescence (Beckman Coulter Inc., Fullerton, CA, USA). Serum AMH levels were assessed in duplicate by an enzyme-linked immunosorbent assay using the AMH/MIS kit (Immunotech, Beckman Coulter, Marseilles, France).
Statistical analysis
The baseline characteristics of the PCOS phenotype were taken on day 2-3 of the menstrual cycle and are expressed as the means ± Standard Deviations (SD). Some of the hormonal concentrations were expressed as a median and range because some of the hormonal variables were not normally distributed. Serum AMH concentrations and continuous variables were presented as medians and ranges. Distributions were compared using the non-parametric Mann-Whitney U-test and Kruskal- Wallis Test. Relationships between AMH and other variables were evaluated by Pearson’s Correlation Coefficient (PCC). P<0.05 was considered significant. Statistical analysis was performed using SPSS, version 17.0 (SPSS Inc.).
Results
Patients’ characteristics
The comparison of the means of BM, BMI, partial of hormonal and metabolic concentrations (PRL, P, FBG, FINS and HOMA-IR) in both groups is shown in Table 1.
| The oligo-ovulation group (N=22) | The anovulation group (N=12) | P-value | |
|---|---|---|---|
| Age | 29.09 ± 3.18 | 29.83 ± 2.48 | 0.808 |
| BW (kg) | 66.19 ± 7.81 | 77.83 ± 12.46 | 0.078 |
| BMI (kg/m2) | 26.35 ± 2.88 | 31.24 ± 4.89 | 0.062 |
| PRL (ng/ml) | 14.84 ± 6.88 | 14.36 ± 6.26 | 0.884 |
| P (ng/ml) | 0.66 ± 0.19 | 0.54 ± 0.26 | 0.35 |
| FBG | 5.43 ± 0.80 | 5.96 ± 0.71 | 0.066 |
| FINS | 21.05 ± 9.75 | 36.2 ± 19.97 | 0.009 |
| HOMA-IR | 5.08 ± 2.58 | 9.28 ± 4.33 | 0.003 |
| Note: BW: Body Weight; BMI: Body Mass Index; FBG: Fasting Blood Glucose; FINS: Fasting Insulin; HOMA-IR: Homeostasis Model Assessment of Insulin Resistance. All statistical analyses were performed using SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL). Data were analysed by two independent samples of non-parametric test and were presented as mean ± SD. P<0.05 was considered significant. | |||
Table 1: Relevant clinical, hormonal, and metabolic features of 34 PCOS women on D2-3 of the menstrual cycle.
There was no noteworthy variation in BM, BMI and age between the groups. There was no significant difference between the groups in FBG (P=0.066). The PCOS women with oligo-ovulation had lower FINS and HOMA-IR values compared with the other group (P=0.009 and P=0.003, respectively).
Characteristics of ovary
On day 2-3 of the menstrual cycle, the OV (ml) and Antral Follicle Count (AFC, both ovaries) were taken and are shown in Table 2. The number of visible antral follicles on both ovaries was higher in the anovulation group than the oligo-ovulation group (P=0.000). The OV (ml) in anovulation group was higher than in the oligo-ovulation group (P=0.000).
| OV (ml) | Antral follicle count (both ovaries) | |
|---|---|---|
| The oligo-ovulation group (N=22) | 10.34 (9.29-12.59) | 29 (25.75-35.00) |
| The anovulation group (N=12) | 17.82 (13.78-22.37) | 41 (37.25-45.0) |
| P -value | 0 | 0 |
| Note: OV: Ovarian per ovary. | ||
Table 2: Ovarian parameters as measured by ultrasound on D2-3 of the menstrual cycle.
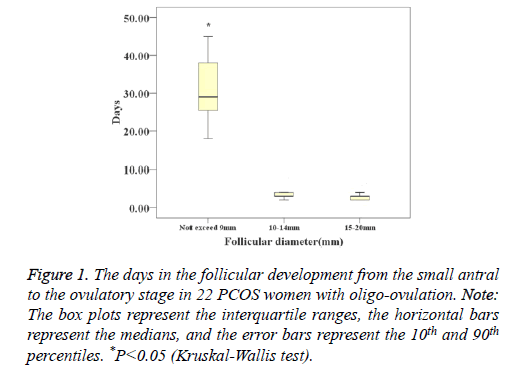
In oligo-ovulation PCOS women, the mean days of ovulation were 37 (28-42.5) days. The days of follicular development in follicles whose diameters did not exceed 9 mm were significantly longer than the days of small follicles whose diameters were 10-14 mm and large follicles whose diameters were 15-20 mm (29 (21-38) days vs. 3.0 (2.5-4) days vs. 3.0 (2.0-3.0) days, P<0.001) (Figure 1).
Figure 1: The days in the follicular development from the small antral to the ovulatory stage in 22 PCOS women with oligo-ovulation. Note: The box plots represent the interquartile ranges, the horizontal bars represent the medians, and the error bars represent the 10th and 90th percentiles. *P<0.05 (Kruskal-Wallis test).
Hormonal parameters
On day 2-3 of the menstrual cycle, serum FSH, E2 and P concentrations showed no differences between the two groups (P>0.05). Serum T and LH concentrations were considerably higher in the anovulation group compared to the oligo-ovulation group (P<0.05).
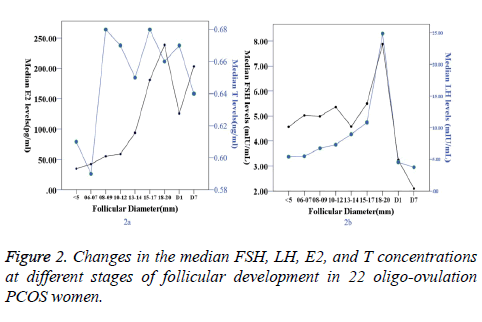
In oligo-ovulation PCOS women, when follicle diameter did not exceed 9 mm, serum E2 concentrations remained at low levels. When the follicle diameter was 10-12 mm, the serum E2 levels began to rise. When the follicle diameter was 18-20 mm, the E2 levels peaked (238.53 (186.04-353) pg/ml). After ovulation, the E2 levels began to decline, with levels of 125.67 (115.18-134.2) pg/ml and 203.44 (185.21-223.36) on Day 1 and Day 7 after ovulation, respectively (Table 3, Figure 2).
| Groups | Time points | AMH (ng/ml) | E2 (pg/ml) | LH (mIU/ml) | FSH (mIU/mL) | T (ng/ml) |
|---|---|---|---|---|---|---|
| The oligo-ovulatory group (N=22) | T1 | 7.46 (5.23-9.92)* | 42.49 (32.70-52.72) | 5.98 (3.76-7.36)* | 4.94 (4.20-5.65) | 0.59 (0.46-0.65)* |
| T2 | 6.30 (4.31-11.75)* | 59.15 (46.69-73.16) | 7.32 (5.69-9.64)* | 5.36 (4.76-5.94) | 0.67 (0.64-0.69) | |
| T3 | 6.77 (5.25-9.64)* | 238.53 (186.04-353)* | 24.85 (18.77-39.01)# | 6.58 (5.67-8.09) | 0.66 (0.47-0.74) | |
| D1 | 125.67 (115.18-134.2)* | 4.55 (3.97-5.60) | 3.24 (2.35-4.65) | 0.67 (0.56-0.70) | ||
| D7 | 203.44 (185.21-223.36)* | 3.74 (2.39-4.35) | 2.09 (1.46-2.89) | 0.64 (0.53-0.7) | ||
| The anovulatory group (N=12) | T4 | 16.8 (11.49-19.74) | 35.14 (22.43-51.09) | 10.52 (8.54-12.36) | 5.17 (4.09-6.53) | 0.79 (0.62-0.89) |
| T5 | 19.96 (17.98-21.93) | 59.32 (44.06-69.97) | 10.36 (8.85-17.02) | 5.00 (4.34-6.54) | 0.78 (0.72-10.4) | |
| Note: P-values were calculated by the Mann-Whitney U test. #Compare to the other groups, P<0.05. *Compare to the T4, T5 group, P<0.05. | ||||||
Table 3: Changes in median (and ranges) AMH concentrations, E2, LH, FSH, T concentrations during menstrual cycles. In oligo-ovulatory PCOS women at five time points: T1=on D2-3 of the menstrual cycle, T2=at selection of the dominant follicle, T3=the time of mature follicle, D1=1 day after ovulation, D7=7 days after ovulation; whereas in anovulatory PCOS women at two time points: T4=on D2-3 of the menstrual cycle, T5=on 60 days or so in the menstrual cycle.
The LH levels showed no difference before follicle diameters had exceeded 14 mm. When the follicle diameter reached 15 mm, the serum LH levels began to rise. The LH levels peaked at a follicle diameter of 18-20 mm (24.85 (18.77-39.01) mIU/ ml). There were no differences in serum FSH and T levels during the process of follicular development. The FSH levels began to decline on Day 1 and Day 7 after ovulation (3.24 (2.35-4.65) and 2.09 (1.46-2.89) mIU/ml) (Table 3, Figure 2).
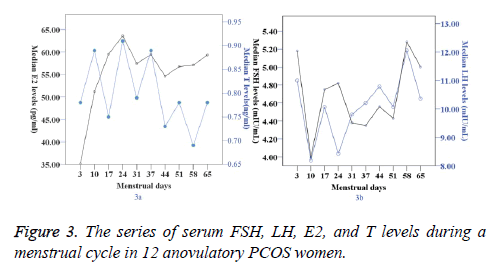
In the anovulation group, the antral follicle had not been activated, and the serum E2 levels fluctuated between 35.14 (22.43-51.09) and 59.32 (44.06-69.97) pg/ml. Serum T and LH concentrations remained in a higher range than in the oligo-ovulation group (P<0.05) (Table 3, Figure 3).
AMH parameters
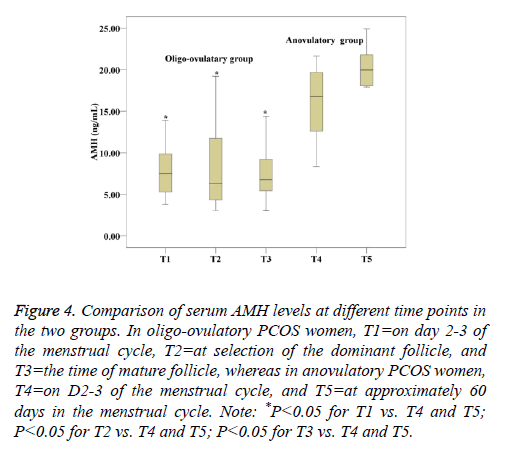
The median AMH level in oligo-ovulation PCOS women is 7.46 (5.23-9.92) ng/ml vs. 16.8 (11.49-19.74) ng/ml in anovulation PCOS women at baseline. Serum AMH concentrations remained constant during the growth and development of antral follicles in oligo-ovulation PCOS women (P>0.05). The results showed that at all time points, women with anovulation PCOS had higher serum AMH concentrations than oligo-ovulation women (P<0.05). There was no significant difference between the two time points in women with anovulation PCOS (P=0.101) (Table 3, Figure 4).
Figure 4: Comparison of serum AMH levels at different time points in the two groups. In oligo-ovulatory PCOS women, T1=on day 2-3 of the menstrual cycle, T2=at selection of the dominant follicle, and T3=the time of mature follicle, whereas in anovulatory PCOS women, T4=on D2-3 of the menstrual cycle, and T5=at approximately 60 days in the menstrual cycle. Note: *P<0.05 for T1 vs. T4 and T5; P<0.05 for T2 vs. T4 and T5; P<0.05 for T3 vs. T4 and T5.
Table 4 showed a correlation coefficient of serum AMH concentrations with different variables at baseline. In the two groups, there is a strong positive correlation between AMH and AFC (P=0.000 and P=0.01, respectively). There is a significant positive correlation between AMH and OV (P=0.013 and P=0.016, respectively) and T (P=0.002 and P=0.028, respectively). There is no correlation between AMH and LH (P=0.248 and P=0.094, respectively).
| Parameters | The oligo-ovulatory group (N=22) | The anovulatary group (N=12) | ||
|---|---|---|---|---|
| PCC | P value | PCC | P value | |
| OV (ml) | 0.522 | 0.013* | 0.673 | 0.016* |
| AFC | 0.809 | 0.000* | 0.71 | 0.01* |
| T (ng/ml) | 0.616 | 0.002* | 0.632 | 0.028* |
| LH (mIU/ml) | 0.257 | 0.248 | 0.506 | 0.094 |
| FINS | 0.033 | 0.883 | 0.124 | 0.7 |
| HOMA-IR | 0.108 | 0.632 | 0.061 | 0.85 |
| Note: PCC: Pearson’s Correlation Coefficient. *P<0.05 | ||||
Table 4: Correlation coefficient of serum AMH concentrations with different variable in 34 PCOS women on D2-3 of the menstrual cycle.
Discussion
Up to 75% of PCOS women are reported to be infertile. Anovulation and oligo-ovulation are important characteristics of PCOS. Forty-eight per cent of the patients showed evidence of follicular development [20]. Our data showed that the mean days of ovulation were 37 (28-42.5) days in oligo-ovulation PCOS women. The activation of antra follicles requires a relatively long platform period and, once antral follicles are activated, they require only a short time to mature.
Circulating FSH levels are thought to be insufficient to reach the increased FSH threshold of the follicle required for selection [21]. Unfortunately, we found that there were no differences in serum FSH levels in the process of follicular development, but serum FSH levels had a slightly increasing trend when follicle diameter was 15-20 mm in our study. In oligo-ovulation PCOS women, serum E2 and LH concentrations remained at lower levels and had a relatively long platform period when follicle diameter did not exceed 9 mm. When the follicle diameter was 10-13 mm, the serum E2 levels began to rise and had a very fast growth trend, which peaked with a follicle diameter of 18-20 mm. When the follicle diameter was 15 mm, the serum LH level began to arise. Serum E2 and LH levels underwent changes throughout the entire period of follicular development. These results show that E2 and LH levels participate in the development of the follicle and the rate of ovulation, and endocrine factors appear to play an important role in the arrest of antral follicle maturation in PCOS.
The majority of women with PCOS have insulin resistance, which is believed to play a fundamental role in the pathogenesis of PCOS [22]. Our data showed that PCOS women with oligo-ovulation had lower FINS and HOMA-IR values compared to the anovulation group. This indicated that higher FINS women with PCOS are more likely to be anovulatory. In summary, endocrine and metabolic factors appear to influence the development of anovulation in PCOS women, but these factors do not exclude the possibility of an intrinsic abnormality of folliculogenesis in PCOS.
Some previous studies provided OV and the 2-5 mm follicle number was positively correlated with androgen levels in the PCOS group [23]. OV is a reflection of the number of small antral follicles present in PCOS, which are the only source of AMH. In our study, the number of visible antral follicles and the OV (ml) in the anovulation group were higher than in the oligo-ovulation group. Turhan et al. suggested the importance of ovarian volume measurement as an indicator of androgen production in PCOS women and reported a significant positive correlation between ovarian volume and serum, LH and T levels [24]. According to our results, there was a positive correlation between AMH, AFC and OV in the two groups, and this result was corroborated by another study [25]. The correlation between AMH, OV and AFC suggests that AMH may be able to replace the ultrasound ovarian morphology criterion in the diagnosis of this syndrome.
The role of AMH in folliculogenesis in humans has not yet been studied in detail. Several studies had shown that AMH expression remains high until a follicle reaches a diameter of approximately 8 mm, and expression is very low in larger antral follicles [26,27]. However, in our study, we found that serum AMH concentrations remained constant during the growth and development of antral follicles in oligo-ovulation PCOS women. Other studies [15,16] have also found that serum AMH concentrations were constant during low-dose ovulation induction in patients with PCOS. This may mean that AMH does not participate in either ovarian primordial follicle recruitment or dominant follicle selection.
In a study by Pellatt et al. AMH production was on average 75 times higher per granulosa cell from anovulatory PCOS and 20 times higher from ovulatory PCOS than healthy ovaries [28]. This result was supported by our study, which found that PCOS women with anovulation showed higher AMH concentrations in different periods of menstruation, possibly due to both the increased number of small antral follicles and intrinsic characteristics of those granulosa cells, which may contribute to anovulation. Our study also showed that serum AMH levels are not affected by the day of the menstrual cycle and are closely correlated with ovulation status. Hence, AMH may be a marker in the diagnosis of PCOS at any time of menstruation. According to the results of this study, it can be stated that high AMH concentrations present in women with PCOS play an integral role in causing anovulation due to AMH’s inhibitory influence on the actions of follicle-stimulating hormones, which normally promote follicular development from the small antral to the ovulatory stage.
Androgen excess in PCOS not only disturbs the delicate balance between androgens, AMH and FSH, but also crucially contributes to ovarian tissue remodeling: stromal hyperplasia and rigidity, hyper vascularity and inflammation. This joint follicular-stromal deregulation is a key mechanism in the pathogenesis of PCOS [29-31]. In our study, serum T levels remained constant in the growth and development of follicles in the oligo-ovulatory group, but circulating T, LH, and AMH levels are significantly higher, which underscores the likely important role of androgens in the dynamics of folliculogenesis in anovulation PCOS women. Pierre et al. found that LH reduces AMHR-II expression in granulosa luteal cells collected from women with normal ovaries and ovulatory PCOS, but not in women with anovulatory PCOS [32]. Although the association between the morphogenesis of the polycystic ovary, androgen excess and elevated serum AMH remains unexplained, serum AMH levels were correlated with the severity of PCOS and specifically with the severity of both hyperandrogenism and oligo-anovulation [33,34]. Our study and others also found a positive correlation between AMH and T in PCOS women with oligomenorrhea [35]. Our results suggested that, while the factors responsible for regulating the spontaneous initiation of follicles remain to unclear, serum T, LH concentration, antral follicles, and OV (ml) may play a role; these findings are also supported by Dissen [36], who found that increased LH secretion, T levels, oocyte-derived peptides, and intraovarian proteins may be implicated in abnormal follicle growth and development. Additionally, a yet undiscovered inherent defect in folliculogenesis may exist.
Conclusions
This study showed that some of PCOS women with oligomenorrhea had spontaneous ovulation; however, the activation of antral follicles of PCOS with oligomenorrhea needs a relatively long platform period, and once antral follicles are activated, they only need a very short time to mature. The factors responsible for aberrant antral follicle development remain unclear, but serum T, LH concentration, antral follicles and OV (ml) may play a role. Constant AMH concentrations during the spontaneous initiation of follicles in oligo-ovulation PCOS women and higher serum AMH concentrations in the anovulation PCOS women suggest that AMH may be used as a marker in the diagnosis or as a predictor of ovulation in PCOS women with oligomenorrhea.
Limitations and Reason for Caution
The number of patients in this study is small. Future research on a larger scale is needed to confirm our findings.
Acknowledgements
We acknowledge Xiaohui Deng who contributed towards the study by making substantial contributions to conception, design, acquisition of data, or analysis and interpretation of data, and Bin Wang was involved in drafting the manuscript or revising it critically for important intellectual content.
Competing Interest
The authors do not have any possible conflicts of interest.
References
- Moran LJ, Hutchison SK, Norman RJ, Teede HJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2011; 1.
- Lauritsen MP, Bentzen JG, Pinborg A. The prevalence of polycystic ovary syndrome in a normal population according to the Rotterdam criteria versus revised criteria including anti-Mullerian hormone. Human Reprod 2014; 29: 791-801.
- Rocca ML, Venturella R, Mocciaro R, Di Cello A, Sacchinelli A. Polycystic ovary syndrome: chemical pharmacotherapy. Expert Opin Pharmacother 2015; 16: 1369-1393.
- El-Gharib MN, Badawy TE. Correlation between insulin, leptin and polycystic ovary syndrome. J Basic Clin Reprod Sci 2014; 3: 49-53.
- Burgers JA, Fong SL, Louwers YV, Valkenburg O, de Jong FH. Oligoovulatory and anovulatory cycles in women with polycystic ovary syndrome (PCOS): whats the difference? J Clin Endocrinol Metab 2010; 95: E485-489.
- Broekmans FJ, Visser JA, Laven JS, Broer SL, Themmen AP. Anti-Müllerian hormone and ovarian dysfunction. Trends Endocrinol Metab 2008; 19: 340-347.
- Li HW, Anderson RA, Yeung WS, Ho PC, Ng EH. Evaluation of serum antimullerian hormone and inhibin B concentrations in the differential diagnosis of secondary oligoamenorrhea. Fertil Steril 2011; 96: 774-779.
- Aghadavod E, Zarghami N, Farzadi L. Evaluation of relationship between serum levels of anti-mullerian hormone, androgen, and insulin resistant with retrieval oocytes in overweight patients with polycystic ovary syndrome. Adv Biomed Res 2015; 4: 76.
- Wiweko B, Maidarti M, Priangga MD, Shafira N, Fernando D. Anti-mullerian hormone as a diagnostic and prognostic tool for PCOS patients. J Assist Reprod Genet 2014; 31: 1311-1316.
- Ali S, Liselotte M, Jurgen H, Walter J, Anupama D. Cut-Off levels of anti-mullerian hormone for the prediction of ovarian response, in vitro fertilization outcome and ovarian hyperstimulation syndrome. Int J Fertil Steril 2015; 9: 157-167.
- Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril 2011; 95: 170-175.
- Saleh BO, Ibraheem WF, Ameen NS. The role of anti-Mullerian hormone and inhibin B in the assessment of metformin therapy in women with polycystic ovarian syndrome. Saudi Med J 2015; 36: 562-567.
- Parahuleva N, Pehlivanov B, Orbecova M, Uchikova E, Ivancheva H. Anti-Mullerian hormone in the major phenotypes of polycystic ovary syndrome. Akush Ginekol (Sofiia) 2014; 53: 22-27.
- Iliodromiti S, Kelsey TW, Anderson RA, Nelson SM. Can anti-Mullerian hormone predict the diagnosis of polycystic ovary syndrome? A systematic review and meta-analysis of extracted data. J Clin Endocrinol Metab 2013; 98: 3332-3340.
- Lie FS, Schipper I, de Jong FH. Serum anti-Mullerian hormone and inhibin B concentrations are not useful predictors of ovarian response during ovulation induction treatment with recombinant follicle-stimulating hormone in women with polycystic ovary syndrome. Fertil Steril 2011; 96: 459-463.
- Fabregues F, Castelo-Branco C, Carmona F,Guimera M, Casamitjana R, Balasch J. The effect of different hormone therapies on anti-Mullerian hormone serum levels in anovulatory women of reproductive age. Gynecol Endocrinol 2011; 27: 216-224.
- The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to Polycystic Ovary Syndrome (PCOS). Hum Reprod 2004; 19: 41-47.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412-419.
- Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update 2003; 9: 505-514.
- Fleming R, Mcqueen D, Yates RWS. Spontaneous follicular and luteal function in infertile women with oligomenorrhoea: role of luteinizing hormone. Clin Endocrinol 1995; 43: 735-739.
- Franks S, Hardy K. Aberrant follicle development and anovulation in polycystic ovary syndrome. Ann Endocrinol (Paris) 2010; 71: 228-230.
- Diamanti-Kandarakis E. Insulin resistance in PCOS. Endocrine 2006; 30: 13-17.
- Koutlaki N, Dimitraki M, Zervoudis S, Poiana C. The relationship between Anti-Mullerian hormone and other reproductive parameters in normal women and in women with polycystic ovary syndrome. J Med Life 2013; 6: 146-150.
- Turhan NO, Senoz S, Gulekli B, Ozaksit G, Oral H, Gokmen O. Clinical and endocrine features of ultrasound diagnosed polycystic ovary patients: the correlation between ovarian volume and androgen activity. J Pak Med Assoc 1993; 43: 4-6.
- Eilertsen TB, Vanky E, Carlsen SM. Anti-Mullerian hormone in the diagnosis of polycystic ovary syndrome: can morphologic description be replaced? Hum Reprod 2012; 27: 2494-2502.
- Weenen C, Laven JS, Von Bergh AR. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Human Reprod 2004; 10: 77-83.
- Jeppesen JV, Anderson RA, Kelsey TW. Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod 2013; 19: 519-527.
- Pellatt L, Hanna L, Brincat M, Galea R, Brain H. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab 2007; 92: 240-245.
- Mcgee WK, Bishop CV, Bahar A. Elevated androgens during puberty in female rhesus monkeys lead to increased neuronal drive to the reproductive axis: a possible component of polycystic ovary syndrome. Hum Reprod 2012; 27: 531-540.
- Lebbe M, Woodruff TK. Involvement of androgens in ovarian health and disease. Mol Hum Reprod 2013; 19: 828-837.
- Falbo A, Rocca M, Russo T, DEttore A, TolinoA, Zullo F, Orio F, Palomba S. Serum and follicular anti-Mullerian hormone levels in women with polycystic ovary syndrome (PCOS) under metformin. J Ovarian Res 2010; 3: 1-6.
- Pierre A, Peigne M, Grynberg M, Arouche N, Taieb J, Hesters L, Gonzales J, Picard JY, Dewailly D, Fanchin R. Loss of LH-induced down-regulation of anti-Mullerian hormone receptor expression may contribute to anovulation in women with polycystic ovary syndrome. Hum Reprod 2013; 28: 762-769.
- Piouka A, Farmakiotis D, Katsikis I, Macut D, Gerou S, Panidis D. Anti-Mullerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: relationship with increased luteinizing hormone levels. Am J Physiol Endocrinol Metab 2009; 296: 238-243.
- Catteau-Jonard S, Bancquart J, Poncelet E, Lefebvre-Maunoury C, Robin G, Dewailly D. Polycystic ovaries at ultrasound: normal variant or silent polycystic ovary syndrome? Ultrasound Obstet Gynecol 2012; 40: 223-229.
- You SY, Park SY, Yang GY, Jeong KA, Kim YJ, Chung HW. Anti-Mullerian hormone in women with polycystic ovary syndrome. Korean J Obstet Gynecol 2012; 55: 315-24.
- Dissen GA, Cecilia GR, Alfonso P. Excessive ovarian production of nerve growth factor facilitates development of cystic ovarian morphology in mice and is a feature of polycystic ovarian syndrome in humans. Endocrinol 2009; 150: 2906-2914.