Research Article - Biomedical Research (2017) Volume 28, Issue 13
Drug Induced Autoimmune Hepatitis (DIAIH): pathological and clinical study
Hui Wang1*, Jindong Fu2, Gang Liu1, Ling Wang2 , Aiguo Yan1 and Gangping Wang3
1Department of Infectious Disease, Rizhao People's Hospital, Rizhao, PR China
2Department of Digestive Disease, Rizhao People's Hospital, Rizhao, PR China
3Department of Pathology, Rizhao People's Hospital, Rizhao, PR China
- *Corresponding Author:
- Hui Wang
Department of Infectious Disease
Rizhao People's Hospital, Rizhao, PR China
Accepted on May 29, 2017
Abstract
Drug-Induced Autoimmune Hepatitis (DIAIH) is a major part of autoimmune hepatitis (AIH). However DIAIH remains poorly characterized, especially how to differentiate DIAIH from AIH in clinical and histology. Moreover, immunosuppression treatment effectiveness has also rarely been discussed so far. In the present study, the clinical data of AIH (n=73) and drug-induced liver injury (DILI) (n=395) in Rizhao People’s Hospital during 2010 to 2014 were retrospective analysed. All AIH patients were diagnosed by simplified diagnosis criteria (2008). DIAIH were drug induced patients with positive autoimmune antibody. The results shown that all 50 AIH cases and 33 DILI cases were identified with available clinical, biochemical and histological data. Eighteen (18/395, 4.55%) of DILI patients were found to be autoimmune antibody positive (named DIAIH). Sex and age at diagnosis of AIH patients were similar to that of DIAIH, ALT, AST and bilirubin levels of DILI and DIAIH patients were significantly higher than those of AIH patients (P<0.05, respectively), and immunoglobulin of AIH patient was significantly higher than that of DIAIH (P<0.05). None of AIH patients could retreat immunosuppression successfully during follow-up, but most DIAIH patients could. AIH is prominent of portal/ portal fibrosis and Rosette formation, interface inflammation and infiltration of plasma cells. With DIAIH, it is mainly consisted by peri-central-venous inflammation and with esophilia cells dominant mixed cells. Conclusion: DIAIH is different from AIH in liver function tests, levels of immunoglobulin and pathological characteristic such as interface hepatitis, plasma cell infiltration, portal fibrosis and Rosette formation. Most DIAIH patients could retreat immunosuppression successfully.
Keywords
Drug-induced autoimmune hepatitis (DIAIH), Autoimmune hepatitis (AIH), Interface hepatitis, Plasma cell infiltration, Immunosuppression, Therapeutic effects, Prognosis.
Introduction
With drug induced immune-mediated liver injury is an immune response against protein antigen within the liver that lead to a syndrome of autoimmune hepatitis (AIH) [1,2]. These antigen created by the liver are mainly from reactive metabolic of some drugs. When they are recognized as neo-antigen by immune system, then liver injury develops [3-5]. Although some case reports about drugs induced autoimmune hepatitis (DIAIH) has been published, such as abolished tienilic acid and in-use nitrofurantion. These cases were firstly reported in Unite d States in 1970’s and the clinical data of them is limited [6,7]. Howeverthere is still few available data on clinicalpathological manifestation and treatment of DIAIH. Moreoverwith some new drugs created, more DIAIH will be founded in clinical works [8-10]. So it is still difficult to differentiate DIAIH from AIH from clinical manifestation for the same positive antibody, especially when DIAIH has no obvert medication history and a mild hepatitis course. And in China, DIAIH is different from western countries, more traditional herbal medicines are in using, which is rarely studied systemically.
Also diagnosis of AIH is a difficult challenge, as clinical spectrum of which is different. And AIH can affect people at various ages, both sexes and different range. Positive antibody is a feature of AIH [11,12]. However, only SLA/LPautoantibodies are specific for the diagnosis of AIH, but they are only present in about 20% of cases. The most common feature in all patients with AIH is an elevation of IgG levels, usually a selective or highly preferential elevation of IgG in comparison to IgA and IgM. However, in some patients the relative increase in IgG levels may be within the normal limits, because the normal range is quite wide [13,14]. Autoantibodies vary, and some patients do not display any autoantibodies at the time of clinical presentation. So in these cases, pathological feature will play an important role in diagnosis of AIH, which will help differentiate AIH from DIAIH.
In this study, we mainly focus on different pathological characteristic of AIH and DIAIH. Meanwhile, we compared clinical, biochemical data and prognosis of DIAIH patients with AIH and drug induce non-AIH patients. Furthermore, we also gave an explicit illustration about treatment strategy of different patients and compare with prognosis or outcome, then gave an evaluation to different treatment strategies.
Materials and Methods
Patients and methods
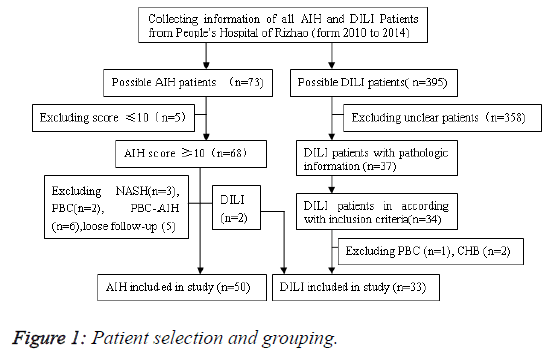
We retrospectively collected all patients with a diagnosis of AIH and drug induced liver injury at Rizhao people’s Hospital from 2010 to 2014 (Figure 1). The study design and procedure were approved by institutional review board of the hospital. All patients were provided written and fully informed consent. Total 309 patients (73 AIH, 236 DILI) were identified. Only patients with available clinical data at baseline and another one follow-up were enrolled. Newly enrolled patients were asked to return to record outcome at 12 months.
Diagnostic criteria
All DILI patients were enrolled if they have a strong history that the liver injury was caused by a medication or an herbal medicine within 6 months before admitted into hospital. Samples were selected based on following criteria before enrolment: (i) Serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level >5 times the upper limit of normal (UNL) or alkaline phosphatase level >2 times the upper limit of normal on 2 consecutive occasion; and (ii) Total serum bilirubin level >2.5 mg/dl along with elevated AST or ALT or alkaline phosphatase level or (iii) International normalized ratio (INR) >1.5 with elevated AST or ALT or alkaline phosphatase level; and (iv) Serum hepatitis, a virus (HAV), hepatitis B virus(HBV), hepatitis C virus(HCV) and hepatitis E virus antibody were negative; and (v) Repeated liver injury with another same medicine using was found (vi) Lymphocyte transformation test or macrophage migration inhibition test was positive. If autoimmune antibody of DILI patients was positive, they were assigned into DIAIH group.
AIH patients in accordance with new simplified criteria proposed by the International Autoimmune Hepatitis Group at baseline were included. The criteria includes the followings which takes as 2 score (i) Globulin, γ-globulin or immunoglobulin G level >1.5 times normal (ii) ANA, SMA, or anti- LKM1 1:80 in adults and 1:20 in children; (iii) No markers of current infection with hepatitis A, B, and C viruses (iv) Histologic features interface hepatitis plasma cells rosettes. Lymphocyte infiltration with no typical interface hepatitis was scored 1; liver disease with other cause such as fatty liver was scored 0. Only patients with score >7 was definite AIH, which was enrolled into this study. Patients with AIH and PBC or PSC overlap syndrome were excluded.
Data of eligible patients were collected from baseline visit and search, during which a medical history and detailed history of liver injury and exposed to implicated agents were obtained. Clinical variables at baseline include age, sex, titers of antinuclear antibody, smooth muscle antibodies, antinuclear cytoplasmic antibodies, liver kidney microsomal, antimitochondrial antibodies, IgG, gamma globulins, aspirate aminotransferases, alanine aminotransferases, alkaline phosphatase, total bilirubin, albumin, international normalized ratio. Also gamma globulins, aspirate aminotransferases, alanine aminotransferases, alkaline phosphatase, total bilirubin, albumin, international normalized ratio were recorded again at start and 1 y after immuno-suppressive treatment. AIH and DIAIH patients were given a liver biopsy and liver biopsy was analysed. Histology were randomly compared between age (± 5 y at diagnosis of AIH and DIAIH ) and sex matched AIH and DIAIH patients.
The overall inflammatory activity (grade) of the whole specimen, portal and interface inflammatory as well as fibrosis (stage) were recorded from available materials on a scale from 0 to 4 and analysed according to review. Interface hepatitis was graded as none, minimal, mild, moderate and severe interface hepatitis and fibrosis stage as no fibrosis, to portal, peri-portal, bridging and cirrhosis. To exactly illustrate interface hepatitis, infiltration of plasma cell, eosinophil cell, lymphocyte, and phagocyte were recorded. Infiltration of plasma cells were graded as 0 none, 1<50 portal district, 2>50 portal district with 6-10 cells per portal district, 3>50 portal district with 11-20 cells per portal district, 4 more than 20 cells per portal. Infiltration of lymphocyte and phagocyte were graded as none, minimal, moderate and active. Additionally, perivenular (Zone 3) necrosis and confluent necrosis were evaluated in biopsy materials review.
Statistical analysis
SPSS 17.0 statistical software was used to analyse the data. Enumeration data with chi-squared test (χ2 test) or Fisher’s exact tests, as appropriate. Continuous variable of the biomarker concentrations are presented as median and interquartile range. The mean ± standard error of mean (SEM), median and other needed values were calculated. The non parametric Mann-Whitney U-test was used to determine differences between two groups. Pathology evaluation were assigned as 3, 6, 9, 12 in accordance with 1-4, intermediate will also be scored. Analysis of pathology score was rank sum test. All tests were two-tailed and conducted by SPSS 17.0. P<0.05 was considered statistically significant.
Results
Clinical features
Only 50 AIH cases and 33 DILI cases were identified with available clinical, biochemical and histological data. And all the patients were definitely diagnosed as previously explicated in methods. Among DILI patients, there are (18/395, 4.55%) patients with positive autoimmune antibodies, namely DIAIH.
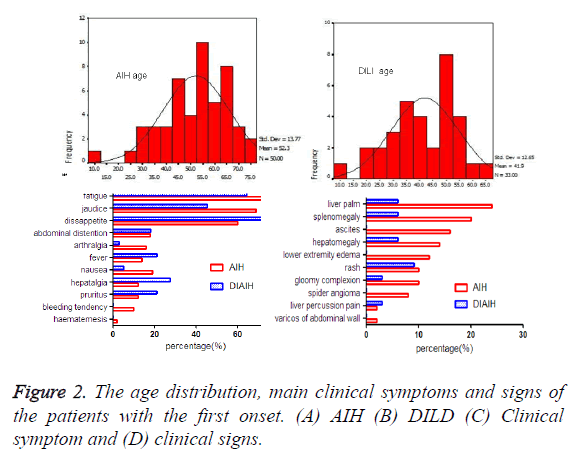
The demographic and biochemical data in the study cohort were demonstrated in Table 1 and Figure 2 Among AIH patients, rate of female was higher than DILI patients. Age at diagnosis or present of AIH group (52.3 ± 13.8) was higher than DILI (41.9 ± 12.7), which was statistically significant. 13 AIH patients were accompanied with other autoimmune disease, such as sjogren syndrome (n=4), rheumatic arthritis (n=4), hyperthyroidism (n=2), hypothyroidism (n=1), autoimmune thyroiditis (n=1), connective tissue disease (n=1). All 7 DIAIH patients had accompanied autoimmune disease, hyperthyroidism (n=2), rheumatic arthritis (n=1), hypothyroidism (n=1), systemic lupus erythematosus (n=1), sarcoidosis (n=1).
| Biological parameters | AIH | DILI | P |
|---|---|---|---|
| Number of cases (n) | 50 | 33 | - |
| Age at diagnosis (y) | 52.3 ± 13.8 | 41.9 ± 12.7 | 0.001 |
| Sex (M/F) | 16893 | 46539 | 0.163 |
| Combine other | 13/50 (26%) | 7/33 (21.2%) | 0.618 |
| Autoimmune disease (%) | |||
| History of allergy (%) | 6/50 (12%) | 7/33 (21.2%) | 0.258 |
Table 1. The clinical information of AIH and DILD.
Liver function tests of AIH patients were different from DILI and DIAIH patients. ALT, AST and ALP levels of DILI and DIAIH patients were higher than that AIH of patients. Though levels of IgG and gamma-globulin of DIAIH patients was elevated, they were still lower than that of AIH patients (Table 2).
| AIH | DILI | P | DIAIH | P | |
|---|---|---|---|---|---|
| (n=50) | (n=33) | (n=18) | |||
| Age | 52.3 ± 13.8 | 41.9 ± 12.7 | 0.098 | 47.8 ± 12.5 | 0.103 |
| ALT (U/L) | 226.74 ± 121.49 | 588.69 ± 455.03 | 0.012 | 548.21 ± 335.29* | 0.021 |
| AST (U/L) | 202.28 ± 111.63 | 439.60 ± 329.73* | 0.023 | 460.46 ± 320.56* | 0.018 |
| ALP (U/L) | 139.82 ± 57.39 | 182.06 ± 88.86* | 0.043 | 176.43 ± 75.01 | 0.051 |
| TBIL (mg/dl) | 2.35 ± 1.52 | 6.98 ± 5.04* | 0.01 | 6.79 ± 5.49* | 0.012 |
| Albumin (g/dl) | 33.39 ± 6.07 | 39.29 ± 6.11* | 0.047 | 37.58 ± 6.01 | 0.053 |
| IgG (<1500 g/dl) | 3594.28 ± 1606.78 | 1733.90 ± 708.83* | 0.028 | 2013.53 ± 790.81* | 0.032 |
| Gamma-globulins (<27 g/l) | 47.84 ± 12.62 | 32.60 ± 7.57* | 0.039 | 35.64 ± 7.84* | 0.042 |
| ANA positive (%) | 46/50 (92) | 18/33 (54.5)* | 0.016 | 17/18 (94.4) | 0.903 |
| SMA positive (%) | 9/50 (18) | 4/33 (12.1) | 0.652 | 4/18 (22.2) | 0.773 |
| ANA and SMA (%) | 9/50 (18) | 4/33 (12.1) | 0.652 | 4/18 (22.2) | 0.773 |
| pANCA | 16/50 (32) | 5/33 (15.2) | 0.236 | 5/18 (27.8) | 0.702 |
| Immunosuppressive therapy (%) | 52/73 (71.2) | 18/33 (57.6) | 0.703 | 18/18 (100) | 0.803 |
| Steroids and azathioprine (%) | 47/52 (90.4) | 5/33 (15.2) | 0.019 | 5/18 (27.8)* | 0.023 |
| Steroid alone | 5/52 (9.6) | 12/33 (35.6) | 0.042 | 12/18 (66.7)* | 0.036 |
| Discontinuation successful (%) | 13/52 (25.0) | 18/18 (100)* | 0.013 | 17/17 (100)* | 0.013 |
| *p<0.05 vs AIH patients. | |||||
Table 2. Comparison of demographic and biochemical, treatment and response in AIH, DILI and DIAIH patients.
Histopathological manifestation
Only part of patients was received liver biopsy at presentation. More AIH patients 54.5% (12/22) had typical histology than DIAIH patients 18.2% (6/33) according to histological characteristic presented by Hennes et al. The typical histological changes of AIH were portal inflammation, interface hepatitis and infiltration of plasma cells. Among them, 86.4% (19/22) patients presented with portal inflammation scores ≥ 3, 59.1% (13/22) patients had piecemeal necrosis scores ≥ 3. Infiltrated cells were prominent with lymphocyte and plasma cells. 54.5% (12/22) patients exhibited intra-lobar inflammation necrosis, such as piecemeal necrosis and confluent necrosis. Infiltration of cells was prominent of lymphocyte, 31.8% (7/22) was plasma cells and 86.4% (19/22) showed various stage of portal fibre formation (Table 3).
| Pathological parameter | AIH (22) | DILD (33) | p | DIAIH (18) | p |
|---|---|---|---|---|---|
| Portal inflammation | 22 (100%) | 24 (72.7%)* | 0.016 | 15 (83.3%)* | 0.021 |
| Piecemeal necrosis | 22 (100%) | 20 (60.6%)* | 0.014 | 14 (77.8%)* | 0.018 |
| Bridged necrosis | 12 (54.5%) | 12 (36.4%) | 0.108 | 9 (50%) | 0.213 |
| Plasma cell Inflammation | 14 (63.6%) | 6 (18.2%)* | 0.017 | 6 (33.3%)* | 0.025 |
| Infiltration of eosinophil cell | 2 (9.1%) | 18 (54.5%)* | 0.013 | 10 (55.6%)* | 0.013 |
| Confluent necrosis | 10 (45.5%) | 8 (24.2%) | 0.094 | 6 (33.3%) | 0.102 |
| Peri-CV necrosis | 5 (22.7%) | 25 (75.8%)* | 0.015 | 15 (83.3%)* | 0.012 |
| Cholestasis | 4 (18.2%) | 13 (39.4%) | 0.207 | 6 (33.3%) | 0.232 |
| Fatty Liver cell | 2 (9.1%) | 13 (39.4%)* | 0.022 | 7 (38.9%)* | 0.023 |
| Massive necrosis | 7 (31.8%) | 5 (15.4%) | 0.205 | 4 (22.2%) | 0.249 |
| Pseudo-lobe Formation | 7 (31.8%) | 0 | 0.056 | 0 | 0.056 |
| Bile duct injury | 4 (18.2%) | 13 (39.4%) | 0.074 | 8 (44.4%) | 0.064 |
| Rosette Formation | 10 (45.5%) | 4 (12.1%)* | 0.034 | 2 (11.1%)* | 0.031 |
| Sinus cell infiltration | 1 (4.5%) | 15 (45.5%)* | 0.012 | 9 (50%)* | 0.009 |
| Phagocyte cell infiltration | 1 (4.5%) | 30 (90.9%)* | 0.006 | 18 (100%)* | 0.001 |
| *p<0.05 vs AIH | |||||
Table 3. Pathological characteristic of liver in AIH, DI-AIH patients.
Although interface hepatitis and portal inflammation were found in DILI patients, cells infiltrated were eosinophil cells and proliferated biliary epithelial cells. More patients 25/33 (75.8%) showed necrosis of central lobe. 39.4% (13/33) patients showed fatty liver changes, All DILI patients showed no pseudo-lobe formation (Table 3) (Figure 3).
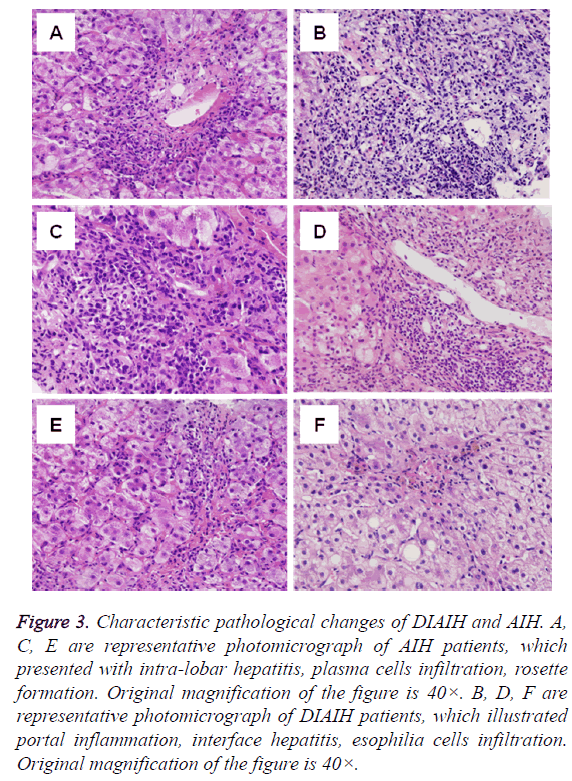
Figure 3: Characteristic pathological changes of DIAIH and AIH. A, C, E are representative photomicrograph of AIH patients, which presented with intra-lobar hepatitis, plasma cells infiltration, rosette formation. Original magnification of the figure is 40×. B, D, F are representative photomicrograph of DIAIH patients, which illustrated portal inflammation, interface hepatitis, esophilia cells infiltration. Original magnification of the figure is 40×.
Inflammation and necrosis scores of AIH and DIAIH patients was no difference (Table 4). But inflammatory cells infiltrated were different, plasma cells in most AIH, esophilia cells mainly in DIAIH (Figure 3).
| Pathological parameter | AIH (22) | DILD (33) | p | DIAIH (18) | p |
|---|---|---|---|---|---|
| Portal inflammation | 22 (100%) | 24 (72.7%)* | 0.016 | 15 (83.3%)* | 0.021 |
| Piecemeal necrosis | 22 (100%) | 20 (60.6%)* | 0.014 | 14 (77.8%)* | 0.018 |
| Bridged necrosis | 12 (54.5%) | 12 (36.4%) | 0.108 | 9 (50%) | 0.213 |
| Plasma cell Inflammation | 14 (63.6%) | 6 (18.2%)* | 0.017 | 6 (33.3%)* | 0.025 |
| Infiltration of eosinophil cell | 2 (9.1%) | 18 (54.5%)* | 0.013 | 10 (55.6%)* | 0.013 |
| Confluent necrosis | 10 (45.5%) | 8 (24.2%) | 0.094 | 6 (33.3%) | 0.102 |
| Peri-CV necrosis | 5 (22.7%) | 25 (75.8%)* | 0.015 | 15 (83.3%)* | 0.012 |
| Cholestasis | 4 (18.2%) | 13 (39.4%) | 0.207 | 6 (33.3%) | 0.232 |
| Fatty Liver cell | 2 (9.1%) | 13 (39.4%)* | 0.022 | 7 (38.9%)* | 0.023 |
| Massive necrosis | 7 (31.8%) | 5 (15.4%) | 0.205 | 4 (22.2%) | 0.249 |
| Pseudo-lobe Formation | 7 (31.8%) | 0 | 0.056 | 0 | 0.056 |
| Bile duct injury | 4 (18.2%) | 13 (39.4%) | 0.074 | 8 (44.4%) | 0.064 |
| Rosette Formation | 10 (45.5%) | 4 (12.1%)* | 0.034 | 2 (11.1%)* | 0.031 |
| Sinus cell infiltration | 1 (4.5%) | 15 (45.5%)* | 0.012 | 9 (50%)* | 0.009 |
| Phagocyte cell infiltration | 1 (4.5%) | 30 (90.9%)* | 0.006 | 18 (100%)* | 0.001 |
Table 4. Pathological score of liver in AIH, DIAIH patients.
Prognosis
In all AIH patients, 52 out of 73 patients received immunosuppressive treatment. Other 21 patients were not suitable for immuno-suppressive treatment for accompanying complications such as ascites, liver failure, upper digestive tract haemorrhage and patient’s institution. 75% patients achieved complete response which is defined as that all liver function tests were normalized within 1 y and sustained at least 6 months, or liver biopsy found least liver inflammation. 10% patients gained part response, 15% relapsed and retreated treatment for reverse contradictions. All DIAIH patients responded well for all treatment, and all can be discontinued treatment in 3-17 months without severe complications.
Discussion
Previous literature on DIAIH mostly was case report and case series. No previous study has been able to assess proportion of DIAIH out of DILI [6-8,10]. In our study, proportion of DIAIH in DILI was firstly reported in clinical aspect. 12.4% of DILI patients were found to be autoimmune antibody positive (namely DIAIH). So DIAIH patients make up a significant proportion of DIAIH patients, which were confused with AIH patients. In DIAIH patients, suspected associated drugs were Chinese traditional medicine, diclofenac, meloxicam and antibiotics, which were different form aboard studies and demographic dependent [7,9,10].
Also in this study, we compared clinical and pathological data of DIAIH and AIH. We found that sex and age at diagnosis of AIH patients were similar to that of DIAIH, which were mostly female and at 55-60. This is different from Japanese study, which showed age at diagnosis of AIH was at 20’s and 50’s [14]. Also this may be one cause that most patients at diagnosis in this study were present with cirrhosis [15]. This is influenced by demography and genetic factor. However, liver function test results showed that ALT, AST and bilirubin levels of DILI and DIAIH patients were significantly higher than that of AIH patients, which may be related with different mechanism [14]. In DILI and DIAIH patients, major mechanism was drug induce direct hepatocyte injury apart from autoimmune antibody induced cell injury. Although antibodies of DIAIH patients were serum positive, their titers were not as high as that of AIH. Immunoglobulin of AIH patient was significantly higher than that of DIAIH [16,17].
In our study, part of AIH patients were not received immunosuppressive treatment for disease associated complications such as liver cirrhosis and ascites [18]. About 50% AIH patient responded to immunosuppressive drug and most treatment were steroids and azathioprine combination strategy. However, none of AIH patients could retreat immunosuppression successfully during follow-up. 50% patients would relapsed after withdraw treatment. Converse with AIH patients, most DIAIH could discontinue treatment successfully in limited time and most of treatment strategy was steroids only, which may be also related with stopping using suspected medicine. Levels of antibodies and immunoglobulin were related with disease progressions, so surveillance of them will contribute to patient management.
Although AIH and DIAIH were serum positive, pathological characteristic of them were different, which facilitated differentiation of them. In this study, we can see that different histological points were following: (1) AIH is prominent of portal, interface inflammation and infiltration of plasma cells. With DIAIH, it is mainly consisted by peri-central-venous inflammation and with esophilia cells dominant mixed cells [18,19]. (2) AIH patients had rare liver fatty degeneration and cholestasis in liver biopsy, but DIAIH is commonly present with liver steatosis and bile thrombus formation in hepatocyte and phagocyte [4,16]. (3) Various stages of sinus and portal fibrosis were shown in AIH liver biopsy which reflecting chronicity of AIH, versus no fibrosis in DIAIH rather than acute necrosis [19,20]. (4) Rosette formation was common in AIH not DIAIH, which was phenomenon of liver cell regeneration. Recently, more cases report that intra-lobe necrosis is also a histological characteristic of AIH without portal inflammation, and follow-up of these patients will develop into typical AIH histology.
So intra-lobe necrosis is assumed to be the early histological changes of AIH [21]. In Japan, authors have found that intralobe necrosis was a predictor of acute disease progression. In this study, 5 cases showed intra-lobe necrosis. Among them, 4 were acute presentation, 1 developed acute disease progression, which was in incidence with previous studies [21].
As DIAIH is drug induced liver autoimmune injury, history of medication is also important point for diagnosis which is different from AIH patients. In China, Chinese traditional medicine is a major cause, with other diclofenac, meloxicam. In west, NSAID drugs and immunosuppression’s are major causes. Also drug induced AIH is an acute occasion, however AIH is mostly a chronic and concealed onset. All these difference will help differentiation of AIH and DIAIH.
All in all, we can see that AIH and DIAIH are similar in autoimmune antibodies positive and in inflammation score. However there is still difference in some aspects such as levels of serum antibody, AST level, immunoglobulin and pathological changes. From histological aspects, we can see that more plasma cells infiltration of portal in AIH can be seen, but more liver steatosis and esophilia cells in DIAIH. All these lead to different treatment strategy and prognosis. AIH patient should be treated with longer combination treatment and less discontinuation chance, DIAIH can be treated with steroids only and can be stopped in limited time.
Conclusion
This finding suggests that DIAIH is different from AIH in liver function tests, levels of immunoglobulin and pathological characteristic such as interface hepatitis, plasma cell infiltration, portal fibrosis and Rosette formation. Most DIAIH patients could retreat immunosuppression successfully.
Conflict of Interest
The authors confirm that this article content has no conflict of interest.
Ethical Issue
The study protocol was endorsed by the Medical Ethical Committee of the Rizhao People's Hospital, besides, each participant provided informed consent.
Acknowledgement
We thank Professor Wang Tailing in Chinese-Japan Friendship Hospital for provision of the pathological staining and evaluation of liver fibrosis.
References
- Castiella A, Zapata E, Lucena MI, Andrade RJ. Drug-induced autoimmune liver disease: A diagnostic dilemma of an increasingly reported disease. World J Hepatol 2014; 6: 160-168.
- Leise, MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc 2014; 89: 95-106.
- Beaune PH, Bourdi M. Auto-antibodies against cytochromes P-450 in Drug-Induced Autoimmune Hepatitis (DIAH). Ann N Y Acad Sci 2010; 685: 641-645.
- Bjornsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, Neuhauser M, Lindor K. Drug-induced autoimmune hepatitis: clinical characteristic and prognosis. Hepatol 2010; 51: 2040-2048.
- Liu ZX, Kaplowitz N. Immune-mediated drug-induced liver disease. Clin Liver Dis 2002; 6: 755-774.
- Fagrell B, Strandberg I, Wengle B. A nitrofurantoin-induced disorder simulating chronic active hepatitis: a case report. Acta Med Scand 1976; 199: 237-239.
- Goldsterin NS, Bayati N, Silverman AL, Gordon SC. Minocycline as a cause of drug-induced autoimmune hepatitis: report of four cases and comparison with autoimmune hepatitis. Am J Clin Pathol 2000; 114: 591-598.
- Bjornsson E, Kalaitzakis E, Av Klinteberg V, Alem N, Olsson R. Long-term follow-up of patients with mild to moderate drug-induced liver injury. Aliment Pharmacol Ther 2007; 26: 79-85.
- Bjornsson E, Davidsdottir L. The long-term follow-up after idiosyncratic drug-induced liver injury with jaundice. J Hepato 2009; 50: 511-517.
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J. Causes, clinical features and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterol 2008; 135: 1924-1934.
- Hennes EM, Zeniya M, Czaja AJ, Pares A, Dalekos GN, Krawitt EL. Simplified criteria for diagnosis of autoimmune hepatitis. Hepatol 2008; 48: 169-176.
- Kessler WR, Cummings OW, Eckert G, Chalasani N, Lumeng L, Kwo PY. Fulminant hepatic failure as the initial presentation of acute autoimmune hepatitis. Clin Gastroenterol Hepatol 2004; 2: 625- 631.
- Czaja AJ. Performance parameters of the diagnostic scoring systems for autoimmune hepatitis. Hepatol 2008; 48: 1540-1548.
- Miyake Y, Iwasaki Y, Terada R, Onishi T, Okamoto R, Takaguchi K, Ikeda H, Makino Y, Kobashi H, Sakaguchi K, Shiratori Y. Clinical features of Japanese type-1 autoimmune hepatitis patients with zone III necrosis. Hepatol Res 2007; 37: 801-805.
- Feld JJ, Dinh H, Arenovich T, Marcus VA, Wanless IR, Heathcote EJ. Autoimmune hepatitis: effect of symptoms and cirrhosis on natural history and outcome. Hepatol 2005; 42: 53-62.
- Czaja AJ. Clinical features, differential diagnosis and treatment of autoimmune hepatitis in the elderly. Drugs Aging 2008; 25: 219-239.
- Strassburg CP, Manns MP. Autoantibodies and autoantigens in autoimmune hepatitis. Semin Liver Dis 2002; 22: 339-352.
- Luth S, Herkel J, Kanzler S, Frenzel C, Galle PR, Dienes HP, Schramm C, Lohse AW. Serologic markers compared with liver biopsy for monitoring disease activity in autoimmune hepatitis. J Clin Gastroenterol 2008; 42: 926-930.
- Hofer H, Oesterreicher C, Wrba F. Centrilobular necrosis in autoimmune hepatitis: a histological feature associated with acute clinical presentation. J Clin Pathol 2006; 59: 246-249.
- Edward LK. Autoimmune hepatitis. N Engl J Med 2006; 354: 54-66.
- Iwai M, Jo M, Ishii M, Mori T, Harada Y. Comparison of clinical features and liver histology in acute and chronic autoimmune hepatitis. Hepatol Res 2008; 38: 784-789.