- Biomedical Research (2014) Volume 25, Issue 4
Does multiple freezing and thawing cycles of serum affect the detection of anti-nuclear antibodies and anti-neutrophil cytoplasmic antibodies by indirect immunofluorescent method?
Melek Demir*, Nural Cevahir, İlknur Kaleli, Nesrin Buluş, Fatma Aydeniz Ozansoy, Özgür Döne YiğitPamukkale University, School of Medicine, Department of Medical Microbiology, Denizli-TURKEY
- *Corresponding Author:
- Melek Demir
Pamukkale University, The School of Medicine
Department of Medical Microbiology
Kinikli 20070, Denizli-TURKEY
Tel: +0 90 258 296 24 89
Fax: +0 90 258 296 17 65
E-mail: mdemir@pau.edu.tr
Accepted date: July 17 2014
Abstract
The aim of this study was to determine whether more than once freezing and thawing of serum affects different patterns of anti-nuclear antibodies (ANA) and anti-neutrophil cytoplasmic antibodies (ANCA) as also to determine for how many days can serum samples be reliably refrigerated (2-8ºC) for these tests. A total of 20 ANA (6 homogenous, 3 speckled, 3 centromere, 2 nucleolar, 2 cytoplasmic and 4 mixed) and 6 ANCA (3 PR-3 positive cANCA, 2 MPO positive pANCA, one MPO negative pANCA) positive serum samples were evaluated. The ANA and ANCA tests were studied with the indirect immunofluorescent (IIF) method using commercial kits. The effect of freezing and thawing was evaluated by comparing frozen serum samples with fresh samples during 5 freezing and thawing cycles. In addition, we analyzed the effect of storage time in the refrigerator. In none of the ANA positive serum samples, except for one mixed pattern, was negativity found in the HEp-2 and liver cells after the freezing and thawing cycles and the 21 day refrigerated period. In ANCA positive serum samples, positivity similar to initial findings continued in all samples during five cycles in freeze/thaw group and at the end of 21 days, in the refrigerated group. It may be concluded that ANA and ANCA can be reliably analyzed in serum samples with five freezing and thawing cycles and after refrigeration up to 21 days.
Keywords
Anti-nuclear antibody, anti-neutrophil cytoplasmic antibody, freezing-thawing, stability, indirect immunofluorescence
Introduction
In autoimmune diseases, autoantibodies develop against various self-antigens found in the nucleus, nucleolus, cytoplasm and cell membrane. Anti-nuclear antibodies (ANA) are the most studied autoantibodies [1-6]. The detection of ANA is important for the diagnosis of various connective tissue diseases [1-3,6]. However, low titers (1:40-1:80) of ANA may be seen in healthy subjects also, particularly in pregnant women and elderly persons. Approximately 40 fluoroscopic ANA types have been defined. Homogenous, speckled, centromere, nucleolar and nuclear dots are the most common nuclear patterns [3]. Anti-neutrophil cytoplasmic antibodies (ANCA) are the autoantibodies that develop against antigenic structures like proteins and enzymes; which are found in the cytoplasmic granules of neutrophils and monocytes. Detection of ANCA in serum is important for the diagnosis of Wegener’s granulomatosis, microscopic polyangiitis and certain types of vasculitis. ANCA is classified as c-ANCA and p-ANCA according to immunofluorescent pattern involvement [7,8].
Different methods such as indirect immunofluorescent, immunoblot and ELISA are used to detect the autoantibodies in serum. Indirect immunofluorescent (IIF) is a widely used method for investigating the presence of ANA, due to its high sensitivity [2-4]. In the immunofluorescent method, human epithelial (HEp-2) cells and/or liver tissue are used for ANA test and granulocytes are used for ANCA [3].
Pre-analytical errors account for up to 70% of all mistakes made in laboratory diagnostics. There are different types of pre-analytical errors. A source of pre-analytical errors is storage conditions of samples. One of the quality indicators in the pre-analytic phase is improperly stored samples [9-11].
Most ANA and ANCA commercial test kits enable concurrent examination of more than one patient sample on a single slide. Laboratories with limited facilities may choose to examine samples after collecting them from all the patients. Therefore serum samples may be stored in the refrigerator (2- 8°C) or deep freezer until enough samples are collected to run the tests. The recommendations of commercial kits about duration of storage of serum in the fridge vary for autoantibodies. Different kits and guidelines differ in their directions about maximum duration of serum storage, such as, 2 days [12,13], 3 days [4], 4-7 days [14,15], and up to 14 days [16]. Commercially available IIF kits warn against repeated freezing and thawing of samples, as it may affect the protein structure of the antibodies causing their degradation. However, in many laboratories, serum samples are frozen and thawed for a number of reasons. Although studies are available in the literature investigating the effect of freezing and thawing on the detection of viral antibodies [17], viral DNA [18,19], thyroid autoantibodies [20], cytokines in sputum [21] and genomic DNA in whole blood [22]; yet to the best of our knowledge, there are no studies investigating how autoantibodies like ANA and ANCA are affected by freezing and thawing.
The aim of this study was to determine whether freezing and thawing of serum, up to five cycles, affects different patterns of ANA and ANCA, and also, the length of time that serum samples can be reliably refrigerated (2-8ºC) for these tests so as to recommend them in routine laboratory studies.
Material and Methods
In this study, patients’ serum samples, which were tested for routine ANA/ANCA were used. ANA and ANCA tests were studied with the IIF method. Slides coated with HEp-2 and hepatic cells (Mosaic HEp-20-10/liver (monkey)- Euroimmun-Germany) were used for ANA testing and biochip slides coated with granulocytes (Granulocyte Mosaic-EUROPLUS-Euroimmun-Germany) were used for ANCA. Serum samples were diluted in titers of 1:100 for ANA and 1:10 for ANCA and tests were carried out following the guidelines of the manufacturer. The study used serum in which different pattern positives like homogenous, speckled, nucleolar, centromere, mixed, cytoplasmic were detected in HEp-2 cells and in granulocyte-coated biochip slides with cANCA and pANCA positive patient serum.
Investigation of the effects of freezing and thawing and the duration of storage in the fridge (2 - 8 ºC)
Serum samples were included in this study on the day that positivity was detected and were divided into two aliquots. Serum from each pattern group was divided into two Eppendorf tubes one of which was stored at -20 ºC in a deep freezer (Group 1) and another at 2-8 ºC in a normal refrigerator (Group 2). The sera of Group 1 were completely thawed at room temperature and examined for ANA and ANCA at 48-72 hour intervals. Tests were repeated by performing 5 cycles of freezing and thawing. With the sera of Group 2, ANA and ANCA tests were repeated on 3rd, 7th, 10th, 14th and 21st days after the first day of the study.
All antibody measurements were routinely performed under the same conditions by the same persons. All slides were evalauted under immunofluorescent (Eurostar-II) microscope by three different researchers concurrently with positive and negative controls in both groups. Fluorescence intensity was interpreted using qualitative IIF scale [2,3,23] (negative, +, ++, +++ and ++++), and recorded. All digital images of the study were recorded.
Results
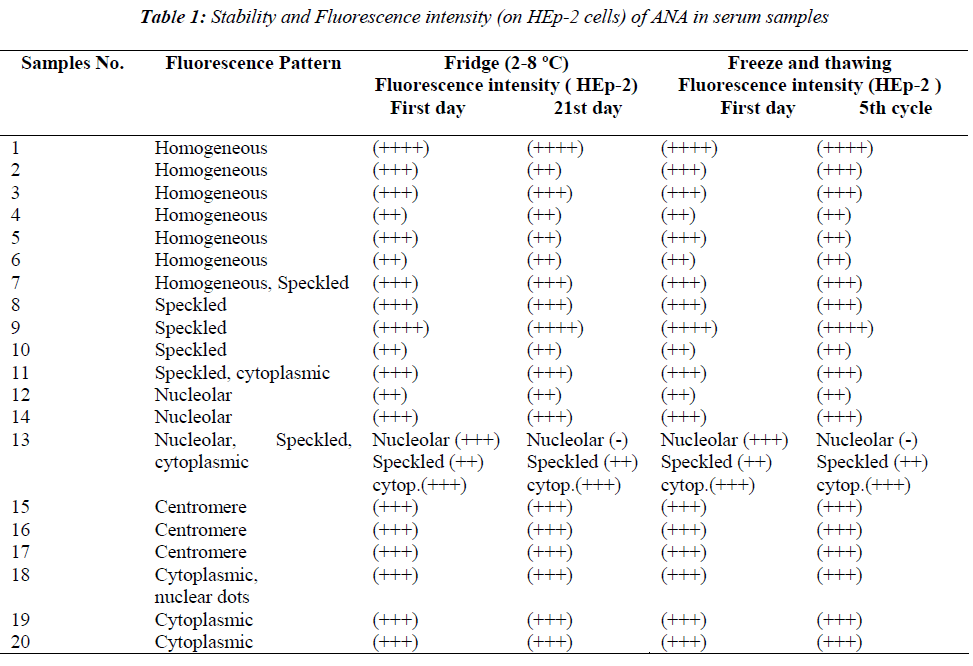
A total of 20 ANA positive serum samples and 6 ANCA positive serum samples were investigated in the study. Of the ANA positive serum, 6 had a homogenous pattern, 3 speckled, 3 centromere, 2 nucleolar, 2 cytoplasmic and 4 with mixed patterns (homogenous-speckled, speckledcytoplasmic, nucleolar-speckled-cytoplasmic, nuclear dotscytoplasmic) (Table 1). Negativity was not observed in any of the homogenous, speckled, centromere, cytoplasmic and mixed patterns, except for one mixed pattern, either after the freezing and thawing procedures or the storing at 2-8 ºC for 21 days, however fluorescent density was reduced one degree in some samples (Table 1).
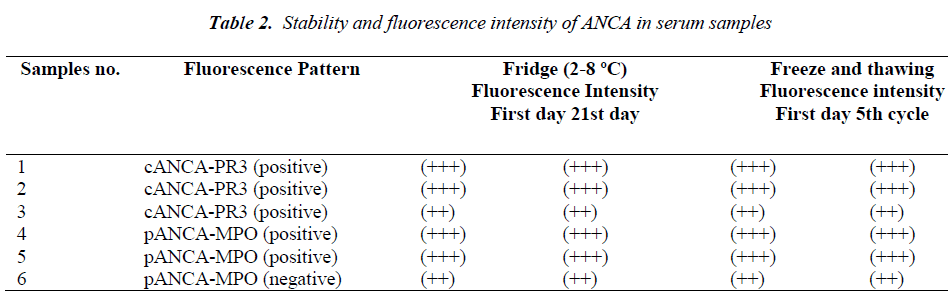
In one mixed pattern (nucleolar-speckled-cytoplasmic) serum sample nucleolar pattern disappeared on 3rd, 7th and 10th days in Group 2, and until fourth cycles in Group 1 on HEp-2 cells, but positivity of speckled and cytoplasmic were found to continue. However nucleolar appearance continued in liver sections of the same sample. In the same serum sample, nucleolar appearance in the HEp-2 cells was re-positive again only on day 14 in the fridge group and in the fourth cycle in the deep freezer group and this nucleolar positivity disappeared in following days and cycles. This serum sample was followed up with three-day intervals up to 30 days and up to 9 freezing and thawing cycles. Although nucleolar positivity continued in the liver cells during this period, the nucleolar was evaluated as negative in HEp-2. The ANA profile test of this serum sample was studied twice (ANA profile 3 EUROLINE- Euroimmun) during this process. AMA-M2 was detected as (+++) positive in both the ANA profile studies and SS-A was detected as a weak positive in the first study. This situation was not observed in the other nucleolar pattern positive serum samples. A total of 6 ANCA positive serum samples were evaluated in the study (Table 2). Of the ANCA positive serum, 3 were PR-3 positive cANCA, 2 MPO positive pANCA and one was MPO negative pANCA. Positivity similar to initial evaluations continued during the five cycles in the deep freezer group and at the end of 21st day in the fridge group. Results consistent with initial findings were obtained at the end of predicted durations in both groups.
Discussion
Pre-analytical errors are usually higher than other laboratory procedures in the total testing process [9]. One of the quality indicators for error in the pre-analytic phase is improperly stored samples [11]. Sometimes serum samples may be stored in the fridge (2- 8°C) or deep freezer until tested. It is a known fact that antibodies keep their stability if serum is stored at below -20 ºC. Although performing more than one freezing and thawing procedure on serum could harm the normal structure of serum and especially lead to the disruption of protein compounds, not many studies are available investigating this issue. In literature, there are different studies investigating whether the freezing and thawing procedure has any effect on detection of antibodies against measles, rubella, mumps viruses in human serum and the detection of HBV-DNA with the PCR method [17-19].
IIF is the most commonly used method for detection of ANA and ANCA. The three parameters required to interpret the ANA test results include, substrate used, titer of the positive test and the fluorescent pattern [2,3]. In this study, serum samples which had different pattern properties were included based on their appearances in HEp-2 cells and hepatocytes. The ANA test results were evaluated from + to ++++ in this study. Except for one mixed pattern serum sample, all samples in both study groups were found to have the same ANA positivity patterns consistent with the initial ones. Only in two serum samples the fluorescence intensity was decreased by one degree. In this study, ANCA positivity was found to continue in both study groups.
In a study investigating the effects of the freezing and thawing procedure on the detection of Suid Herpesvirus 1 antibodies with ELISA in wild boar serum, results were reported to change in 10 of the 37 serum samples at different cycles of the five freezing and thawing cycles [24]. In another study, TSH, fT4, TPO-Ab and TG-Ab were investigated in serum and no statistically significant difference was found between results obtained after 0, 1, 3 and 6 days in samples stored at +4 degrees. In the same study, frozen and fresh serum samples were compared and it was reported that there was no difference between the results with 50 freezing and thawing cycles [20]. In another study investigating the effect of freezing and thawing on measles, mumps and rubella antibodies evaluated by the ELISA method, it was reported that there is no statistically significant difference in IgG antibody levels at the end of 10 freezing and thawing cycles for all three viruses and that the antibodies were quite stable [17].
In our study, it was observed that different ANA pattern positivities were not affected by study conditions, except for one mixed pattern. In one mixed pattern (nucleolarspeckled- cytoplasmic) serum sample, nucleolar pattern disappeared in HEp-2 cells, but in liver sections, nucleolar positivity was observed to continue in both study groups in during study period. AMA-M2 was found to be positive, SS-A was found to be a weak positive in the ANA profile study of this serum sample. The nucleolus is the site in which rRNA precursor molecules (pre-rRNAs) are synthesized, coordinated and accumulated in eukaryotic cells. Various nucleolar proteins and molecules responsible for ribosomal activity have been reported to be localized in different regions of the cell nucleolus in different phases of the cell cycle [25-27]. PM/Scl, nucleolin, fibrillarin, RNAP-I, I/III and human upstream binding factor cause nucleolar pattern appearance in HEp-2 cells [2,3]. Although the ANAprofile test kit used in our study contains anti-PM-Scl, the positivity was not detected in the above mentioned mixt pattern. It was considered that this serum sample might contain an antibody against another antigen group; which could form a nucleolar pattern appearance (anti-RNAP-I, I/III, anti-nucleolin or anti-fibrillarin). In previous studies, particularly anti-RNAP I/III antibodies were reported to show difficult nucleolar staining in HEp-2 cells [28,29].
In our study, it was quite interesting to detect the continuance of nucleolar pattern in liver sections in the above mentioned mixed-pattern positive serum and not in the HEp-2 cells. From the beginning of this study, attention was given to make sure that tests were conducted and evaluated by the same hands and that this study was conducted in a similar fashion as in routine patient studies. Test kits with the same lot number were used and all results were evaluated under the same microscope and interpreted by the same researchers during this study. Therefore, we may conclude that the findings in the mentioned mixed pattern case did not result from study conditions or examiners. In addition, the continuing nucleolar appearance during the study period in liver cells suggests that there was no loss in the antibody titer due to the time of storage in the fridge or the freezing and thawing procedure. This condition was considered to arise from the properties of HEp-2 cells and the antibody relationship causing nucleolar positivity in this serum sample.
In spite of our efforts, a similar study investigating the effects of freezing and thawing and duration of storage at 2-8ºC on ANA and ANCA stability could not be found in the published literature. Thus the results of our study can be considered valuable for providing recommendations for routine laboratory studies.
Conclusions
Based on the results obtained in this experiment, we may conclude that; ANA and ANCAs remained stable and could be detected with IIF in serum samples stored in the refrigerator for up to 21 days and during five freezing and thawing cycles.
Using liver cell series concurrently with HEp-2 cells for ANA could be useful for the detection of some antibodies; which may not be detected in HEp-2 due to various reasons. The immediate examination of autoantibody tests is important for test result quality and accuracy. However, tests may not be done immediately due to laboratory facilities and working conditions. In this study, it was concluded that storing serum samples in a normal refrigerator or a deep freezer does not negatively affect the detection of ANA and ANCA with IIF.
This study was approved by the Clinical Researches Ethics Committee of Pamukkale University Medical Faculty, Turkey.
References
- Muro Y. Antinuclear antibodies. Autoimmunity 2005; 38:3-9.
- Kumar Y, Bhatia A, Minz RW. Antinuclear antibodies and their detection methods in diagnosis of connective tissue diseases: a journey revisited. Diagn Pathol 2009;4:1.
- Tozzoli R, Bizzaro N, Tonutti E, et al. Guidelines for the laboratory use of autoantibody tests in the diagnosis and monitoring of autoimmune rheumatic diseases. Am J Clin Pathol 2002;117:316-24.
- Kavanaugh A, Tomar R, Reveille J, Solomon DH, Homburger HA. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of Pathologists. Arch Pathol Lab Med 2000;124:71-81.
- Tozzoli R. The diagnostic role of autoantibodies in the prediction of organ-specific autoimmune diseases. Clin Chem Lab Med 2008;46:577–87.
- Fritzler MJ, Salazar M. Diversity and origin of rheumatologic autoantibodies.Clin Microbiol Rev 1991;4:256-69.
- Wiik A. What you should know about PR3-ANCA an introduction. Arthritis Res 2000;2:252–4.
- Savige J, Pollock W, Trevisin M. What do antineutrophil cytoplasmic antibodies (ANCA) tell us? Best Pract Res Clin Rheumatol 2005;19:263–76.
- Plebani M. The detection and prevention of errors in laboratory medicine. Ann Clin Biochem 2010;47:101–10.
- Carraro P, Plebani M. Errors in a Stat laboratory: Types and frequencies 10 years later. Clin Chem 2007;53:1338–42.
- Plebani M. Quality indicators to detect pre-analytical errors in laboratory testing. Clin Biochem Rev 2012;33:85-8.
- ANA HEp-2 test system. Available at: http://www.zeusscientific.com/fileadmin/media/pdfs/inserts/ifa/autoimmune/pi/ZEUS%20IFA%20ANA%20HEp-2%20Test%20System-FA2400EBR2022EN.pdf Accessed :19 March 2013.
- HEp-2 ANA Kits/Substrate Slides. Available at: http://www.inovadx.com/PDF/di/708100_EN.pdf Accessed :19 March 2013.
- Antinuclear antibody test system HEp-2. Available at: http://www.trinitybiotech.com/Product%20Documents/10-1240-29EN%20Antinuclear%20Antibody%20Test%20System.pdf Accessed:19 March 2013.
- Anti-nuclear antibodies HEp-2 (ANA-HEp-2). Available at: http://www.orgentec.com/products/pdfs/IFU_IFT_DE_EN/ORG%20870_IFU_EN_QM122130_2012-08-08_1.pdf Accessed :19 March 2013.
- Euroimmun AG. Mosaic HEp-20-10/Liver (monkey). Instruction for indirect immunofluorescence test. FA_1512-1_A_UK_C06.doc Version: 01/03/2011
- Pinsky NA, Huddleston JM, Jacobson RM, Wollan PC, Poland GA. Effect of multiple freeze-thaw cycles on detection of measles, mumps, and rubella virus antibodies. Clin Diagn Lab Immunol 2003;10:19-21.
- Durmaz R, Otlu B, Direkel S. Effect of multiple freezing and thawing of serum on TT virus and hepatitis B virus DNA positivity. Arch Virol 2002:147:515–8.
- Sanlidag T, Akcali S, Ozbakkaloglu B. Serum hepatitis B DNA: stability in relation to multiple freeze–thaw procedures. J Virol Methods 2005;123:49–52.
- Männistö T, Surcel HM, Bloigu A, et al. The effect of freezing, thawing, and short- and long-term storage on serum thyrotropin, thyroid hormones, and thyroid autoantibodies: implications for analyzing samples stored in serum banks. Clin Chem 2007;53:1986-7.
- Tirelli AS, Colombo C, Torresani E, Cariani L, Arnaboldi E, Conese M. Validation of an automatedsensitive immunoassay for quantitation of cytokines in the sputum of cystic fibrosis patients. Clin Chem Lab Med 2007;45:108-11.
- Shao W, Khin S, Kopp WC. Characterization of Effect of Repeated Freeze and Thaw Cycles on Stability of Genomic DNA Using Pulsed Field Gel Electrophoresis. Biopreserv Biobank 2012;10:4-11.
- Carballo OG, Ingénito FB, Ginaca AA, Carabajal P, Costa MA, Balbaryski J. First Argentine consensus for standardization of antinuclear antibodies by indirect immunofluorescence–HEp-2. Acta Bioquím Clín Latinoam 2012;46 :3-13.
- Boadella M, Gortazar C. Effect of haemolysis and repeated freze-thawing cycles on wild boar serum antibody testing by ELISA. BMC Research Notes 2011;4:498.
- Hernandez-Verdun D, Roussel P, Thiry M, Sirri V, Lafontaine DL. The nucleolus: structure/function relationship in RNA metabolism. Wiley Interdiscip Rev RNA 2010;1:415-31.
- Hernandez-Verdun D.Assembly and disassembly of the nucleolus during the cell cycle. Nucleus 2011;2:189-94.
- Fomproix N, Gébrane-Younès J, Hernandez-Verdun D. Effects of anti-fibrillarin antibodies on building of functional nucleoli at the end of mitosis. J Cell Sci 1998;111:359-72.
- Yamasaki Y, Honkanen-Scott M, Hernandez L, et al. Nucleolar staining cannot be used as screning test for the scleroderma marker anti-RNA polymerase I/II antibadies. Arthritis & Rheumatism 2006;54:3051-6.
- Kuwana M, Okano Y, Kaburaki J, Medsger TA, Wright TM. Autoantibodies to RNA polymerases recognize multiple subunits and demonstrate crossreactivity with RNA polymerase complexes. Arthritis & Rheumatism 1999;42:275-84.