Research Article - Biomedical Research (2020) Volume 31, Issue 4
Docosahexaenoic acid attenuates cell death and interleukin-1beta secretion in THP-1 cells responded to Aggregatibacter actinomycetemcomitans invasion
Aki Kawano1, Wataru Ariyoshi1, Ryota Yamasaki1, Yoshie Yoshioka1, Kosuke Kashiwagi2, Daisuke Namikawa3, Tatsuji Nishihara1, Toshinori Okinaga4*
1Division of Infections and Molecular Biology, Department of Health Promotion, Kyushu Dental University, Kitakyushu, 803-8580, Japan
2Department of Fixed Prosthodontics, Osaka Dental University, Osaka, 573-1121, Japan
3Department of Geriatric Dentistry, Osaka Dental University, Osaka, 573-1121, Japan
4Department of Bacteriology, Osaka Dental University, Osaka, 573-1121, Japan
- *Corresponding Author:
- Toshinori Okinaga
Department of Bacteriology
Osaka Dental University
Osaka
Japan
Accepted date: June 01, 2020
Abstract
Macrophages play an important role in the innate immune system by producing inflammatory cytokines in response to substances derived from bacteria and viruses. Recently, omega-3 fatty acids have been shown to have beneficial effects in inflammatory diseases. In this study, we investigated the effects of omega-3 fatty acids on inflammatory responses and cell death induced by the Gram-negative bacterium Aggregatibacter actinomycetemcomitans in macrophages. A. actinomycetemcomitans invasion induced secretion of interleukin (IL)-1β and expression of inflammasome-associated factors, including nucleotide-binding oligomerization domain like receptor protein 3 (NLRP3), adaptor apoptosisassociated speck-like protein containing a caspase recruitment domain (ASC), caspase-1, caspase-4, and cleaved-gasdermin D (GSDMD), in THP-1 cells. Docosahexaenoic acid (DHA), an omega-3 fatty acid suppressed NLRP3, ASC and caspase-1 expression as well as IL-1β secretion by THP-1 cells in response to A. actinomycetemcomitans invasion. Moreover, DHA impaired assembly of ASC, suppressed the expression of caspase-4 and cleaved-GSDMD, and suppressed cell death in A. actinomycetemcomitans-invaded THP-1 cells. Taken together, our data suggest that DHA attenuates the secretion of IL-1β and cell death induced by A. actinomycetemcomitans invasion of macrophages via suppression of inflammasome-associated factors.
Keywords
Docosahexaenoic acid, Aggregatibacter actinomycetemcomitans, Macrophage, Inflammasome, Interleukin-1β, Cell death
Introduction
Aggregatibacter actinomycetemcomitans is a Gram-negative microorganism that is frequently isolated from patients with periodontal disease [1]. In periodontal tissue, A. actinomycetemcomitans induces host immune responses by producing exotoxicand endotoxic virulence factors [2]. Pathogen recognition is essential to activate the innate immune system, and is primarily performed by macrophages and dendritic cells. Periodontopathic bacteria including A. actinomycetemcomitans are medically important pathogens, and many have been associated with systemic disorders such as diabetes mellitus [3], arteriosclerotic cardiovascular diseases [4], and infectious diseases [5]. Chronic systemic inflammation is the result of pro-inflammatory cytokine secretion following activation of the innate immune system. Previous experiments showed that A. actinomycetemcomitans invasion induced production of interleukin (IL)-1β, a critical inflammatory mediator in macrophages [6].
Inflammation is an essential component of immune responses and can be triggered by tissue damage or bacterial infection. The early events of the innate immune response are mediated by pattern recognition receptors (PRRs) [7]. The inflammasome is a large intracellular signalling complex containing cytosolic PRRs including nucleotide-binding oligomerization domain-like receptors (NLRs) [8]. In NLR inflammasome complexes, the NLR protein 3 (NLRP3) inflammasome plays a notable role in innate immune responses to bacterial infection [9]. The NLRP3 inflammasome, through the adaptor apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), activates caspase-1 resulting in processing and release of IL-1β. IL-1β is a key cytokine for control of immune tolerance during inflammation [10]. These pathways are currently designated as “canonical” and “non-canonical” NLRP3 inflammasome activation [11]. Canonical inflammasome activation requires two independent steps: transcription and oligomerization. In the first step, LPS is recognized by TLR4, promoting NLRP3 inflammasome transcription following nuclear factor kappalight- chain-enhancer of activated B cells (NF-κB) activation. NLRP3 inflammasome oligomerization results in IL-1β release at the second step [12]. By contrast, non-canonical inflammasome activation in macrophages is induced by Gramnegative bacteria such as Escherichia coli, Citrobacterrodentium or Vibrio cholerae[13]. In the noncanonical inflammasome pathway, LPS released by cytosolic Gram-negative bacteria, which is endocytosed by host cells, activates mouse caspase-11 or human caspase-4/5 [14]. Activation of caspase-4 promotes production of proinflammatory cytokines and results in cleavage of the newly discovered protein, gasdermin D (GSDMD), which is required for pyroptosis[15]. Pyroptosis is a type of inflammatory programmed cell death and is regulated via caspase-1- dependent or caspase-1-independent mechanisms. Activation of inflammatory caspases causes pyroptosis as well as IL-1β, these functions are also important for eliminating pathogens and maintaining host cell homeostasis [16].
Fatty acids modulate inflammatory processes and contribute to the pathophysiology of diet-related chronic diseases [17]. Fatty acids are composed of carbon, hydrogen, and oxygen, and their physiological activity differs depending on the number of double bonds [18]. Polyunsaturated fatty acids (PUFAs) are classified as essential fatty acids that cannot be synthesized by mammals and therefore must be obtained from dietary sources. There are two main categories of PUFAs: omega-6 and omega-3 PUFAs. Several human nutritional studies have suggested that dietary factors can promote or reduce inflammation [19,20]. Diets high in fat, especially saturated fatty acids appear to increase the risk of developing heart disease [21]. By contrast, foods high in omega-3 PUFAs can have beneficial effects in rheumatoid arthritis and in stabilizing advanced atherosclerotic plaques [22]. Previously, we reported that docosahexaenoic acid (DHA), an omega-3 PUFA, promoted M2 macrophage polarization [23]. M2 macrophages have anti-inflammatory properties mediated through secretion of anti-inflammatory cytokines. However, the relationships between inflammatory responses induced by bacterial infection and omega-3 PUFAs are poorly understood.
In the present study, we examined the ability of A. actinomycetemcomitans to induce expression of inflammatory cytokines and non-canonical inflammasome related factors in macrophages. We show that DHA suppressed macrophage inflammatory responses by inhibiting ASC speck formation, secretion of IL-1β, and prevented macrophage pyroptosis induced by A. actinomycetemcomitans invasion via suppression of the non-canonical inflammasome pathway.
Materials and Methods
Cells
THP-1, human leukemiamonocytic cell lines were purchased from the Japanese Cancer Research Resources Bank (Osaka, Japan). These cells were cultured according to published methods [23]. To differentiate THP-1 monocytes into macrophages, cells were stimulated with phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich) overnight in 6-well plates (Iwaki, Chiba, Japan). And then, cells were refreshed to the culture medium [24]. After 48 h, cells were pre-treated with docosahexaenoic (DHA) (50 μM) for 24 h.
Bacterial strains and A. actinomycetemcomitans invasion
A. actinomycetemcomitans strain Y4 was grown according to the method accounted by previous report [6]. THP-1 cells (5×105 cells/mL) were invaded with A. actinomycetemcomitans at a multiplicity of infection (MOI) of 50 in 6-well plates (Iwaki) according to the procedure described by Okinaga et al. By using this method, it was confirmed that bacteria can invade the cells under a microscope [6].
Reagents
DHA was purchased from Cayman Chemical (Ann Arbor, MI, USA). Anti-IL-1β polyclonal antibody was from Novus Biologicals (Centennial, CO, USA). Anti-NLRP3 monoclonal antibody was purchased from AdipoGen Life Sciences (Basel, Switzerland). Anti-caspase-1 monoclonal antibody was from Abcam (Cambridge, UK), and anti-caspase-4 monoclonal antibody was from MBL (Nagoya, Japan). Anti-ASC and anti- GSDMD monoclonal antibodies were from Santa Cruz Biotechnology (Dallas, TX, USA). Anti-β-actin monoclonal antibody was obtained from Sigma-Aldrich (St. Louis, MO, USA). Anti-rabbit anti-IgG horseradish peroxidase (HRP) conjugated antibody and anti-mouse anti-IgG HRP conjugated antibody were obtained from GE Healthcare (Little Chalfont, UK).
Quantitative real time PCR (qRT-PCR)
qRT-PCR was performed in accordance with a published protocol (Kawano et al., 2019). The primer sequences for qRTPCR: human IL-1β, forward 5ʹ- TCAGCCAATCTTCATTGCTCAA-3 ′ and reverse 5ʹ- TGGCGAGCTCAGGTACTT CTG-3 ’ ; human β-actin, forward 5ʹ-GCGCGG CTACAGCTTCA-3ʹ and reverse 5ʹ- CTTAA TGTCACGCACGATTTCC-3ʹ.
Enzyme-linked immunosorbent assay (ELISA)
Cell supernatants were assessed for human IL1-β and mouse IL1-β using Quantikine ELISA kits (R&D systems) according to manufacturer’s instructions.
Western blotting
Proteins from treated cells were extracted using sodium dodecyl sulfate (SDS) lysis buffer (50 mMTris – HCl containing 2% SDS, pH 6.8) and a protease inhibitor mixture (Thermo Fisher Scientific, MA, USA). Western blotting was performed as described in our previous report.
Cell viability and cytotoxicity assays
To evaluate cell viability, a cell counting kit-8 (CCK-8) (Dojindo Molecular Technologies, Kumamoto, Japan) was used. The CCK-8 assay was performed according to manufacturer’s protocol. Cytotoxicity was assessed using a lactate dehydrogenase (LDH) assay kit (Dojindo Molecular Technologies). The assay was performed with recovered supernatants according to the manufacturer’s protocol.
Immunofluorescence
Cells were fixed using the method accounted by Okinaga et al. [6]. The cells were incubated with anti-ASC antibody overnight at 4°C and incubated with secondary Alexa Fluor 488-conjugated goat anti-mouse (Invitrogen, Carlsbad, CA, USA) for 1 h at room temperature. The cells were mounted in VECTASHIELD mounting medium with DAPI (Vector Laboratories, Burlingame, CA, USA). All-in-One Fluorescence microscope system (Keyence, Tokyo, Japan) was used for detection of immunofluorescence images.
Statistical analyses
Data were analysed using Excel (Microsoft, Redmond, WA, USA) and expressed as the means ± standard deviations. Oneway ANOVA test were used followed by Tukey’s post hoc test. The difference between two groups was assessed using unpaired Student’s t-tests. A value of p<0.05 was considered statistically significant.
Results
DHA suppressed production of IL-1β induced by A. actinomycetemcomitans invasion
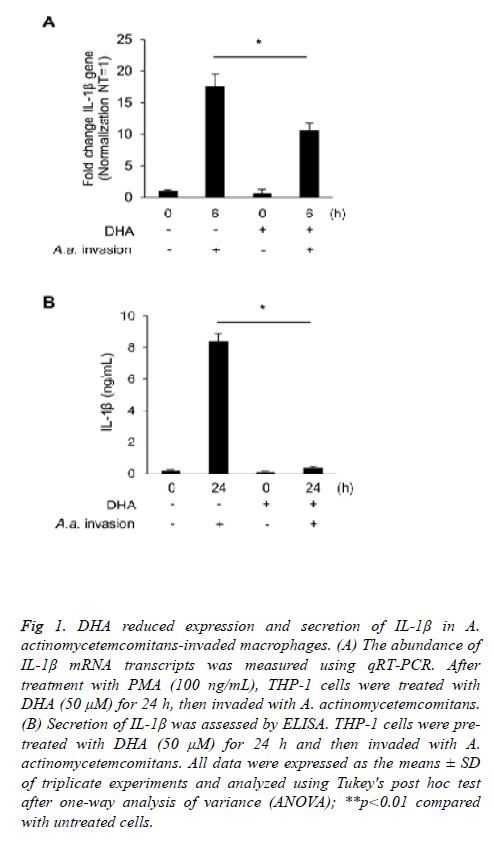
We used PMA-differentiated THP-1 macrophages to investigate the inflammatory effects of A. actinomycetemcomitans invasion. A. actinomycetemcomitans enhanced the expression of IL-1β 6 h post-invasion of THP-1 macrophages; however, DHA pre-treatment suppressed upregulation of IL-1β transcripts (Figure 1A). ELISA showed that DHA significantly downregulated IL-1β secretion 24 h post-invasion with A. actinomycetemcomitans in THP-1 macrophages (Figure 1B). To confirm the effect of other fatty acids on secretion of IL-1β, we used EPA, another omega-3 fatty acid, and PAL, a saturated fatty acid. EPA slightly reduced the secretion of IL-1β induced by A. actinomycetemcomitans invasion, while PAL increased.
Figure 1: DHA reduced expression and secretion of IL-1β in A. actinomycetemcomitans-invaded macrophages. (A) The abundance of IL-1β mRNA transcripts was measured using qRT-PCR. After treatment with PMA (100 ng/mL), THP-1 cells were treated with DHA (50 μM) for 24 h, then invaded with A. actinomycetemcomitans. (B) Secretion of IL-1β was assessed by ELISA. THP-1 cells were pretreated with DHA (50 μM) for 24 h and then invaded with A. actinomycetemcomitans. All data were expressed as the means ± SD of triplicate experiments and analyzed using Tukey's post hoc test after one-way analysis of variance (ANOVA); **p<0.01 compared with untreated cells.
DHA suppressed ASC speck formation
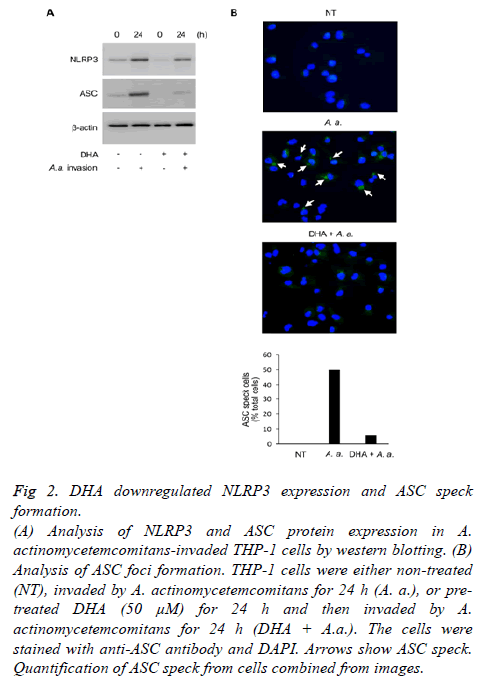
To explore the effects of DHA on inflammasome activity, we investigated the expression of NLRP3 and ASC. As shown Figure 2A, DHA suppressed NLRP3 and ASC expression induced 24 h following A. actinomycetemcomitans invasion. In addition, DHA inhibited the assembly of ASC 24 h following A. actinomycetemcomitans invasion (Figure 2B). A. actinomycetemcomitans promoted ASC speck formation in about 50% of the cells, but was rescued by treatment with DHA.
Figure 2: DHA downregulated NLRP3 expression and ASC speck
formation.
(A) Analysis of NLRP3 and ASC protein expression in A.
actinomycetemcomitans-invaded THP-1 cells by western blotting. (B)
Analysis of ASC foci formation. THP-1 cells were either non-treated
(NT), invaded by A. actinomycetemcomitans for 24 h (A. a.), or pretreated
DHA (50 μM) for 24 h and then invaded by A.
actinomycetemcomitans for 24 h (DHA + A.a.). The cells were
stained with anti-ASC antibody and DAPI. Arrows show ASC speck.
Quantification of ASC speck from cells combined from images.
DHA reduced the expression of caspase-1 and suppressed the non-canonical inflammasome pathway
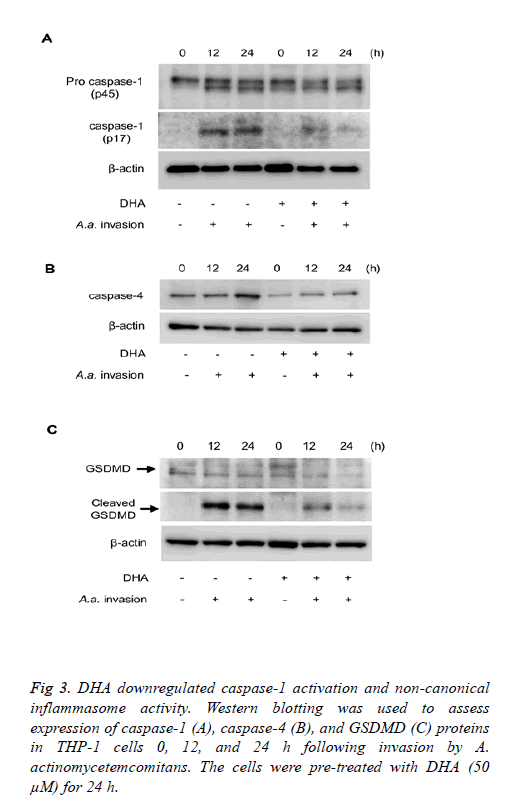
The influence of DHA on expression of caspases important for inflammasome activity was investigated. DHA treatment suppressed caspase-1 activation induced by A. actinomycetemcomitans invasion for 12-24 h (Figure 3A). We also investigated the effect of DHA on non-canonical inflammasome signalling. As shown in Figure 3B, expression of caspase-4 induced 12-24 h following A. actinomycetemcomitans invasion was inhibited by DHA pretreatment. In addition, A. actinomycetemcomitans invasion for 12-24 h induced cleavage of GSDMD, cleavage was suppressed by DHA pre-treatment (Figure 3C). The roles of pro-inflammatory mediators during A. actinomycetemcomitans invasion were investigated using siRNA-transfected THP-1 cells. Cells independently silenced for ASC, caspase-1, caspase-4 and GSDMD expressions were prepared. IL-1β secretion by A. actinomycetemcomitans-invaded THP-1 cells was reduced in the absence of ASC, caspase-1, and GSDMD.
Figure 3: DHA downregulated caspase-1 activation and non-canonical inflammasome activity. Western blotting was used to assess expression of caspase-1 (A), caspase-4 (B), and GSDMD (C) proteins in THP-1 cells 0, 12, and 24 h following invasion by A. actinomycetemcomitans. The cells were pre-treated with DHA (50 μM) for 24 h.
DHA attenuated A. actinomycetemcomitans cytotoxicity
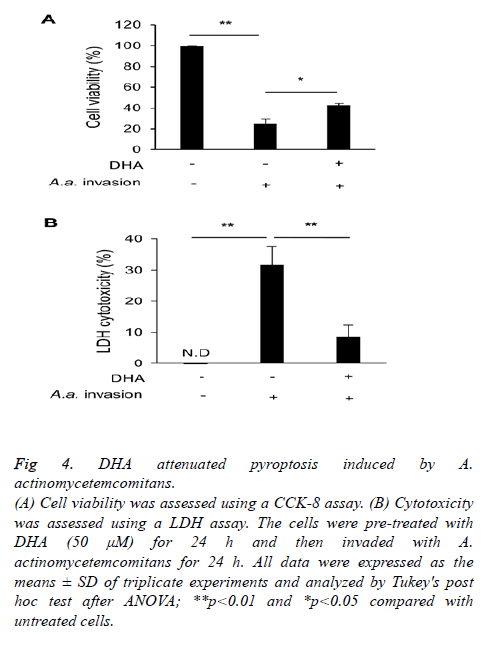
Finally, we examined the cell damage induced by A. actinomycetemcomitans and its mitigation by DHA. A. actinomycetemcomitans invasion reduced cell viability and stimulated the release of LDH, but DHA pre-treatment suppressed both effects (Figures 4A and 4B).
Figure 4: DHA attenuated pyroptosis induced by A.
actinomycetemcomitans.
(A) Cell viability was assessed using a CCK-8 assay. (B) Cytotoxicity
was assessed using a LDH assay. The cells were pre-treated with
DHA (50 μM) for 24 h and then invaded with A.
actinomycetemcomitans for 24 h. All data were expressed as the
means ± SD of triplicate experiments and analyzed by Tukey's post
hoc test after ANOVA; **p<0.01 and *p<0.05 compared with
untreated cells.
Discussion
In this study, we showed that DHA suppressed cell death and the secretion of IL-1β via reduction of ASC speck formation and the expression of non-canonical inflammasome-associated factors following A. actinomycetemcomitans invasion of macrophages. Our results may be useful in establishing novel treatment strategies for chronic oral inflammatory diseases using DHA supplementation.
Aggressive periodontitis is primarily caused by A. actinomycetemcomitans invasion and excessive inflammatory responses in immune cells, including macrophages [25]. We previously reported that A. actinomycetemcomitans invasion of macrophages occurred through a phagocytic process and that cytotoxicity was linked to cytoplasmic factors in macrophages [6]. We showed that DHA suppressed the response to A. actinomycetemcomitans invasion in the human monocytic cell line THP-1 (Figure 1A). As shown Figure 1B, secretion of IL-1β into culture media was increased by A. actinomycetemcomitans invasion. Thus, it was shown that DHA has an effect of suppressing IL-1β gene expression and extracellular secretion.
Omega-3 fatty acids, which are abundant in fish oil, suppress the functions of lipid inflammatory mediators, such as prostaglandins or leukotrienes, derived from omega-6 fatty acids [26]. Furthermore, foods rich in omega-3 fatty acids can have beneficial effects in inflammatory diseases such as diabetes and atherothrombotic cardiovascular disease [26-28]. In recent years, several studies of omega-3 fatty acids and their effects on the inflammasome have been conducted. Omega-3 fatty acids inhibit the expression of NLRP3 via G-protein coupled receptor (GPR) 40 and GPR120 [29]. In addition, DHA inhibited NLRP3 inflammasome assembly in a myeloidderived suppressor cell line [30]. These results support our finding that DHA reduced NLRP3 and ASC expression following A. actinomycetemcomitans invasion (Figure 2A). ASC, an important constitutive protein in all inflammasomes, acts as an adapter that helps link PRRs with caspase-1 in inflammasomes [31]. When the inflammasome is formed, ASC aggregates to form a complex called “ASC specks”. Hence, ASC specks can be used as an upstream indicator of inflammasome activation. As shown in Figure 2B, DHA inhibited speck formation induced by A. actinomycetemcomitans invasion. Therefore, DHA may inhibit signal transduction by directly affecting ASC-associated upstream events in inflammasome formation. Another study showed that dietary PUFAs suppressed NLRP3 inflammasome formation through autophagy using knockout experiments of autophagy-related factors [32,33]. We previously reported that DHA promoted the differentiation of M2 macrophages with anti-inflammatory activity through autophagy-related mechanisms [23]. Considering this finding, it is possible that autophagy is also partially caused by degradation of NLRP3 and ASC by DHA. By contrast, PAL, a saturated fatty acid, enhanced IL-1β secretion. PAL can promote polarization of specific cells in response to inflammatory stimuli [34]. In addition, PAL attenuates autophagy mechanisms and is involved in development of insulin sensitivity and obesityrelated metabolic diseases such as type 2 diabetes [35]. Proinflammatory M1 macrophages accumulated in pancreatic islets in a mouse model of type 2 diabetes, and expression of inflammatory cytokines such as IL-1β and tumor necrosis factor-α was also enhanced [36]. Therefore, PAL shows effects opposite to those of the PUFAs DHA and EPA. Further research is needed to investigate whether fatty acids regulate inflammasome activity triggered by autophagy-associated molecules.
Caspase-1 activation in macrophages invaded with Salmonella or Shigella species results in cytokine processing and cell death [37]. Caspases-4 and -5, which belong to the family of cysteine proteases, are classified as inflammatory caspases. These enzymes contain a long prodomain containing a caspase activation and recruitment domain (CARD) and are composed of large and small subunits. Human caspase-4 is a regulator of non-canonical inflammasome activation and contributes to defense against Gram-negative bacteria [38]. GSDMD is a critical target of caspase-4 and a key mediator of host responses [39]. The N-terminal fragment of GSDMD mediates pore formation in the plasma membranes of dying cells [40]. Caspase-4 binds directly to cytosolic LPS via its CARD. LPS binding induced oligomerization of caspase-4, indicating that the noncanonical pathway was activated [41]. In our experiments, DHA significantly reduced the expression of caspase-1, caspase-4, and cleaved-GSDMD induced by A. actinomycetemcomitans invasion (Figure 3). These results indicated that DHA prevents cell membrane damage caused by Gram-negative bacterial invasion through suppression of caspase-4-mediated GSDMD cleavage. Thus, we conclude that cleavage of GSDMD was blocked directly or indirectly by DHA treatment. IL-1 family cytokines are released during inflammatory responses in numerous tissues [42]. IL-1β is detected as a cytosolic factor that lacks an N-terminal secretion signal and is therefore not secreted via conventional pathways [43]. These facts suggest that membrane pore formation by the N-terminal fragment of GSDMD is important in extracellular release of IL-1β. Hence, DHA may inhibit the formation of cell membrane pores caused by the cleavage of GSDMD. Furthermore, we speculate that DHA may subsequently block the non-canonical inflammasome signaling pathway by inhibiting the interaction between A. actinomycetemcomitans and caspase CARDs. We confirmed that ASC, caspase-1, and GSMDD silencing inhibited IL-1β secretion, but caspase-4 silencing had no effect (data not shown). In the absence of caspase-4, it is considered that IL-1β secretion is promoted by A. actinomycetemcomitans via the canonical pathway that does not require caspase-4.
Inflammatory programmed cell death, called pyroptosis, can be classified as canonical or non-canonical based on the involvement of inflammatory caspases (caspase-1 and caspase-4 in human cells) [38]. Interestingly, we found that A. actinomycetemcomitans induced caspase activation and cytotoxicity in macrophages that was suppressed by DHA treatment (Figure 4). To assess the effect of DHA on cytotoxicity induced by A. actinomycetemcomitans invasion, we used a LDH assay. LDH is an enzyme present in the cytoplasm that is released into cell culture medium when plasma membranes are damaged, and used to pyroptosis detection [44]. DHA has been reported to affect lipid micro domains in the cell membrane, called “lipid rafts,” that play a role in immune cell signalling pathways important for inflammation [45]. One report indicated that the saturated fatty acid lauric acid promoted TLR4 recruitment to lipid rafts following LPS treatment, but that DHA inhibited this effect. Moreover, the effect of DHA on the physical properties of plasma membrane micro domains has been demonstrated in various cell types [46]. As shown in Figure 4B, DHA suppressed release of LDH caused by membrane damage following invasion by A. actinomycetemcomitans in THP-1 cells, suggesting that DHA exerted a protective effect on the cell membrane.
Conclusion
In summary, we assessed the effects of DHA on inflammatory responses induced by A. actinomycetemcomitans invasion of human macrophages. DHA suppressed IL-1β secretion via downregulation of ASC assembly. Furthermore, DHA inhibited the non-canonical inflammasome pathway by downregulating caspase-4 and subsequent cleaved-GSDMD. Therefore, DHA suppressed cleavage of GSDMD resulting in inhibition of pyroptosis. Thus, DHA can suppress the inflammatory response by altering inflammasome signaling and inhibit pyroptosis by preventing cleavage of GSDMD. These findings demonstrate DHA’s inhibitory effects on secretion of IL-1β and cell death during bacterial invasion. DHA may have beneficial effects on the inflammatory responses caused by periodontal pathogens.
Acknowledgements
This work was partially supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 18K09556 and 18K09797). We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
References
- Bhattacharjee MK, Childs CB, Ali E. Sensitivity of the periodontal pathogen Aggregatibacter actinomycetemcomitans at mildly acidic pH. J Periodontol 2011;82:917-925.
- Herbert BA, Novince CM, Kirkwood KL. Aggregatibacter actinomycetemcomitans, a potent immunoregulator of the periodontal host defense system and alveolar bone homeostasis. Mol Oral Microbiol 2016;31:207-227.
- Castrillon CA, Hincapie JP, Yepes FL, Roldan N, Moreno SM, Contreras A, Botero JE. Occurrence of red complex microorganisms and Aggregatibacter actinomycetemcomitans in patients with diabetes. J Investig Clin Dent 2015;6:5-31.
- Straka M, Kazar J, Pijak MR, Gasparovic J, Wsolova L, Mongiellova V. The importance of the presence of Aggregatibacter actinomycetemcomitans in sulcus gingivalis of patients with cardiovascular diseases. Med SciMonit 2011;17:CR646-649.
- Kang J, de BritoBezerra B, Pacios S, Andriankaja O, Li Y, Tsiagbe V, Schreiner H, Fine DH, Graves DT. Aggregatibacter actinomycetemcomitans infection enhances apoptosis in vivo through a caspase-3-dependent mechanism in experimental periodontitis. Infect Immun 2012;80:2247-2256.
- Okinaga T, Ariyoshi W, Nishihara T. Aggregatibacter actinomycetemcomitans invasion induces interleukin-1beta production through reactive oxygen species and cathepsin B. J Interferon Cytokine Res 2015;35:431-440.
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010;140:805-820.
- Latz E, Xiao T.S. Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol 2013; 13:397-411.
- Elinav E, Henao-Mejia J, Flavell RA. Integrative inflammasome activity in the regulation of intestinal mucosal immune responses. Mucosal Immunol 2013;6:4-13.
- Gagliani N, Palm NW, de Zoete MR, Flavell RA. Inflammasomes and intestinal homeostasis: regulating and connecting infection, inflammation and the microbiota. IntImmunol 2014;26:495-499.
- Pellegrini C, Antonioli L, Lopez-Castejon G, Blandizzi C, Fornai M. Canonical and non-canonical activation of NLRP3 inflammasome at the crossroad between immune tolerance and intestinal inflammation. Front Immunol 2017;8:36.
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 2009;183:787-791.
- Kayagaki N, Warming S, Lamkanfi M, VandeWalle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. Non-canonical inflammasome activation targets caspase-11. Nature 2011;479:117-121.
- Yi YS. Regulatory roles of the caspase-11 non-canonical inflammasome in inflammatory diseases. Immune Netw 2018;18:e41.
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015;526:660-665.
- Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 2017;277:61-75.
- Simopoulos AP. Essential fatty acids in health and chronic disease. Forum Nutr 2003;56:67-70.
- Tomer KB, Crow FW, Gross ML. Location of double-bond position in unsaturated fatty acids by negative ion MS/MS. J Am ChemSoc 1983;105:5487-5488.
- Basu A, Devaraj S, Jialal I. Dietary factors that promote or retard inflammation. ArteriosclerThrombVascBiol 2006;26:995-1001.
- Calder PC, Ahluwalia N, Albers R, Bosco N, Bourdet-Sicard R, Haller D, Holgate ST, Jönsson LS, Latulippe ME, Marcos A, Moreines J, M'Rini C, Müller M, Pawelec G, van Neerven RJ, Watzl B, Zhao J. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br J Nutr 2013;109:S1-34.
- Briggs MA, Petersen KS, Kris-Etherton PM. Saturated fatty acids and cardiovascular disease: replacements for saturated fat to reduce cardiovascular risk. Healthcare 2017;5:29.
- Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. BiochemSoc Trans 2017;45:1105-1115.
- Kawano A, Ariyoshi W, Yoshioka Y, Hikiji H, Nishihara T, Okinaga T. Docosahexaenoic acid enhances M2 macrophage polarization via the p38 signaling pathway and autophagy. J Cell Biochem 2019;120:12604-12617.
- Chanput W, Mes JJ, Wichers HJ. THP-1 cell line: an in vitro cell model for immune modulation approach. IntImmunopharmacol 2014;23:37-45.
- Kim S, Park MH, Song YR, Na HS, Chung J. Aggregatibacter actinomycetemcomitans-induced AIM2 inflammasome activation is suppressed by xylitol in differentiated THP-1 macrophages. J Periodontol 2016;87:e116-126.
- Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes. Br J Clin Pharmacol 2013;75:645-662.
- Chen C, Yu X, Shao S. Effects of omega-3 fatty acid supplementation on glucose control and lipid levels in type 2 diabetes: a meta-analysis. PLoS One 2015;10:e0139565.
- Mason RP. New insights into mechanisms of action for omega-3 fatty acids in atherothrombotic cardiovascular disease. Curr Atheroscler Rep 2019;21:2.
- Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, Guarda G, Tian Z, Tschopp J, Zhou R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013;38:1154-1163.
- Dumont A, de Rosny C, Kieu TL, Perrey S, Berger H, Fluckiger A, Muller T, Pais de Barros JP, Pichon L, Hichami A, Thomas C, Rébé C, Ghiringhelli F, Rialland M. Docosahexaenoic acid inhibits both NLRP3 inflammasome assembly and JNK-mediated mature IL-1β secretion in 5-fluorouracil-treated MDSC: implication in cancer treatment. Cell Death Dis 2019;10.
- Bryan NB, Dorfleutner A, Rojanasakul Y, Stehlik C. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J Immunol 2009;182:3173-3182.
- Shen L, Yang Y, Ou T, Key CC, Tong SH, Sequeira RC, Nelson JM, Nie Y, Wang Z, Boudyguina E, Shewale SV, Zhu X. Dietary PUFAs attenuate NLRP3 inflammasome activation via enhancing macrophage autophagy. J Lipid Res 2017;58:1808-1821.
- Williams-Bey Y, Boularan C, Vural A, Huang NN, Hwang IY, Shan-Shi C, Kehrl JH. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-kappaB activation and enhancing autophagy. PLoS One 2014;9:e97957.
- Riera-Borrull M, Cuevas VD, Alonso B, Vega MA, Joven J, Izquierdo E, Corbí ÁL. Palmitate conditions macrophages for enhanced responses toward inflammatory stimuli via JNK activation. J Immunol 2017;199:3858-3869.
- Hernandez-Caceres MP, Toledo-Valenzuela L, Díaz-Castro F, Ávalos Y, Burgos P, Narro C, Peña-Oyarzun D, Espinoza-Caicedo J, Cifuentes-Araneda F, Navarro-Aguad F, Riquelme C, Troncoso R, Criollo A, Morselli E1. Palmitic acid reduces the autophagic flux and insulin sensitivity through the activation of the free fatty acid receptor 1 (FFAR1) in the hypothalamic neuronal cell line N43/5. Front Endocrinol 2019;10.
- Eguchi K, Manabe I, Oishi-Tanaka Y., Ohsugi M, Kono N, Ogata F, Yagi N, Ohto U, Kimoto M, Miyake K, Tobe K, Arai H, Kadowaki T, Nagai R. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell Metab 2012;15:518-533.
- Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasinSipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA 1999;96:2396-2401.
- Yuan YY, Xie KX, Wang SL, Yuan LW. Inflammatory caspase-related pyroptosis: mechanism, regulation and therapeutic potential for inflammatory bowel disease. Gastroenterol Rep 2018;6:167-176.
- Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015;526:666‐671.
- Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends BiochemSci 2017;42:245-254.
- Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014;514:187-192.
- Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 2018;48:35-44.
- Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity 2013;39:1003-1018.
- Rayamajhi M, Zhang Y, Miao EA. Detection of pyroptosis by measuring released lactate dehydrogenase activity. Methods Mol Biol 2013;1040:85-90.
- Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem 2009;284:27384-27392.
- Stillwell W, Shaikh SR, Zerouga M, Siddiqui R, Wassall SR. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod Nutr Dev 2005;45:559-579.