Research Article - International Journal of Pure and Applied Zoology (2021) Volume 9, Issue 8
DIVERSITY OF AMPHIBIAN FAUNA AND THEIR ROLE IN BIOLOGICAL CONTROL OF INSECT PESTS AT CAUVERY DELTA REGIONS OF TAMIL NADU
A. Veeramani*, B. Kumaresan
PG and Research Department of Zoology, Government Arts College (Autonomous), Kumbakonam – 612002, Tamil Nadu, India
- Corresponding Author:
- A. Veeramani
PG and Research Department of Zoology,
Government Arts College (Autonomous),
Kumbakonam – 612002,
Tamil Nadu,
India
E-mail: wildveera@gmail.com
Received: 03th November, 2021; Accepted: 17th November, 2021; Published: 24th November, 2021
Abstract
Amphibians are mostly habitat specific and are highly sensitive vertebrates. So, these are called indicator species of environment and also, they play an important role in ecological cycle of the agricultural fields because they feed on insects, including many pest species of agricultural crops. They are also important food sources for many larger animals such as water birds, mammals, reptiles, and even spiders and larger insects. The amphibian fauna of the Cauvery delta region is less explored as compared to other regions like the Western Ghats range. The Cauvery belt regions has variety of crop pattern such as paddy, Cotton, sugar cane, maize, plantain, black gram, groundnut, etc. provides the ideal environment and habitats for the occurrence of amphibians. Apart from that there are natural and artificial wetlands such as ponds, pools, rivers tributaries of river Cauvery and few lakes. Such habitats are well attracted to amphibian species and may use of various purposes such as food, breeding and home ground etc. Changing of crop pattern, road kills, conversion of cultivable lands and urbanization are seriously affecting the diversity of amphibian fauna directly and indirectly. The amphibians are playing an important role of act as an agent for biological control of insect pests at cultivable lands and controlling larvae during its tadpole stage. Little studies has been initiated so far the amphibians in the study area. The Road transect method was applied for the estimation of abundance of amphibians. All Anurans species were recorded by direct sighting method and also by recording the calls from the concerned species. Dead or road killed specimens of amphibians were collected for examine the gut content to know the food remains. A total of 212 individuals of amphibians belonging to 10 species, 7 genera and 4 families were recorded from different areas of the study area. This forms the first scientific document of the amphibians of the study area. The chi-square test of amphibians recorded in the study area shows that there is significant differences among the species X2=59.5094, df =9, p=0.000.
Keywords
Amphibian, Food habits, Diversity, Biological control, Insect pest, Cauvery delta.
Introduction
Amphibians are belonging to the class Amphibia that are called herpetofauna of vertebrate. The term Amphibians is derived from Greek words “amphibious” for their dual life style (Amphi-dual; Bios-life). Amphibians are characterized by their ability to live both aquatic and terrestrial habitats. Some of the species are permanent as land dwellers, while some of other species have a completely aquatic mode of life. Amphibians are classified into three Orders namely; 1. Anura (Greek: An-absent, Oura–Tail) includes frogs and toads. 2. Caudata (Greek: Cauda–Tails) includes Newts and Salamanders and 3. Gymnophina (Greek: Gymno–Naked, Ophios–snake) includes Caecilians. All the amphibians of the above orders are closely dependent on water, especially for their breeding purpose. Anuran includes frogs and toads which are belonging to the genus Bufo are described as toads where as members of genus Rana are referred as frogs. In the order Caudata (literally meaning tailed Amphibians) there are no clear distinctions between the two categories of Newts and Salamanders, both common names are often interchanged (Kanaujia and Kumar, et al., 2013) [1].
Amphibians are habitat specific and highly sensitive vertebrates. So, these are called indicator species of environment and also, they play an important role in ecological cycle of the agricultural fields (Blaustein and Wake, et al., 1990; Vitt et al., 1990; Wyman, et al., 1990; Wake, et al., 1991; Cushman, et al., 2006; Karunakaran and Jeevanandham, et al., 2017) [2-7]. Amphibians currently comprising of more than 7301 recognized species in the world and 342 species found in India (Frost, et al., 2013). Out of the known Amphibian species from India, 75 species are yet to be evaluated and 81 species are still under the data deficient category (Dinesh et al., 2013) [8]. Out of the 342 species of amphibians found in India which includes 306 species of anurans, 35 species of Gymnophionas and 1 species of salamander (Dinesh et al. 2013). The amphibians are diverse and unique, with more than 80% of the 77 amphibian species being endemic from the state of Tamil Nadu, India (Dinesh and Radhakrishnan, et al., 2009) [9]. Also, many new species have recently been discovered from India, especially in Western Ghats (Vasudevan and Dutta et al., 2000; Dutta and Ray, et al., 2000; Biju and Bossyut, et al., 2003; Gururaja et al., 2007; Dinesh et al., 2008; Biju et al., 2009, 2010; Joshy et al., 2009 [10-16]). Amphibians are more threatened and declining in population than birds and mammals (Stuart et al., 2004) [17].
To implement conservation programmes for amphibians it is important to understand the factors that control their diversity in the region. Amphibians play an important role in the ecosystem because they feed on insects, including many pest species of agricultural crops. They are also important food sources for many larger animals such as water birds, mammals, reptiles, and even spiders and larger insects. They often have the role of economical importance to humans as a food source (Mazzoni et al., 2003; Daszak et al., 2004 [18-19]), medical resource in some regions (Chinese medicine) (Zhou et al., 2006) [20], and as an important potential source of future pharmaceutical drugs (Clarke, et al., 1997) [21]. Most of the endemic species have restricted distribution, confined to the rainforests of the Western Ghats (Vasudevan et al., 2001) [22]. This tropical region is covered by large expanses of brooks, swamps, ponds and farm lands all of which have considerable amount of vegetation, breeding ground for amphibians. This area greatly supports the amphibian diversity and provides suitable shelter for the different species of amphibians.
Amphibians are have two life stages namely tadpoles (occur in water) and adults (on land). It comprised of frogs, toads, caecilians and salamanders those are extremely varied in shape and size. The amphibian fauna of the Cauvery delta region is less explored as compared to other regions like the Western Ghats range. The Cauvery belt regions has variety of crop pattern such as paddy, Cotton, sugar cane, maize, plantain, black gram, groundnut, etc. provides the ideal environment and habitats for the occurrence of amphibians. Apart from that there are natural and artificial wetlands such as ponds, pools, rivers tributaries of Cauvery and few lakes. Such habitats are well attracted to amphibian species and may use of various purposes such as food, breeding and home ground etc. Changing of crop pattern, road kills, conversion of cultivable lands and urbanization are seriously affecting the diversity of amphibian fauna directly and indirectly.
Amphibians are in the midst of an extinction crisis. According to the Global Amphibian Assessment, nearly one- third of all amphibian species are endangered or threatened, making amphibians the most endangered group of animals in the world. The rapid disappearance of amphibian populations in the recent decades has become undoubtedly the most tragic loss of biodiversity, and it is one of the most serious environmental issues. Alteration and destruction of both terrestrial and aquatic habitats are the largest threats. Research of past two decades has proved amphibian declination is mainly due to water pollution. The water may appear clean but there is enormous physico-chemical elements dissolved in it, in which it contaminates water and affect the quality of water and life (Thenmozhi and Karthik, et al., 2016) [23].
However, this region supports a dense human population, mainly associated with agricultural activities, which impose severe anthropogenic pressures on the natural biotic communities (Karthik, et al., 2017) [24]. Amphibians are generalist feeders that prey on any moving prey that so happened to cross their line of vision. The natural diet of amphibians includes a wide spectrum of insects and other invertebrates such as annelids, arachnids, millipedes, and molluscs (Ahmad, et al., 2009; Diett et al., 2009; Hirai and Matsui, et al., 2002; Nurul and Ibrahim, et al., 2008; Santos et al., 2004; Sole et al., 2009; Toft, et al., 1981) [25-29]. Larger amphibians are also known to prey on small mammals, birds and even other amphibians. The feeding strategies of the amphibians depend on their degree of specialization. Anurans can be narrow-mouthed, poisonous, active foragers, ant-specialists, non-ant specialists, opportunistic, wide-mouthed, cryptic, sit and-wait foragers or a simple generalist that has no speciation in foraging mode or type of prey.
Amphibians such as frogs and toads only target moving prey and prefer elongated prey such as crickets or insect larvae that move across their field of vision. However, many aquatic amphibians will target food by scent and will consume inert food. The suitability and range of live feeds are assessed in the Amphibian Population Management Guidelines (Schad, et al., 2007) [30]. Because we cannot simulate the natural diet of many wild amphibians, that often eat 100’s to 1000’s of prey daily, we have to provide nutrition using a few invertebrate species of relatively large size (McWilliams, et al., 2008) [31].
The amphibians are playing an important role of act as an agent for biological control of insect pests at cultivable lands and controlling larvae during its tadpole stage. There are about 217 species of amphibians have been reported from Western Ghats of India (Dinesh and Radhakrishnan, et al., (2011), Biju et al., (2011), Anil et al., (2011a & 2011b), Dinesh et al., (2011) [32- 35]. In Cauvery delta region of Mannampandal, Mayiladuthurai there were 13 species of amphibians were reported by Karthik et al., (2017), 14 species by Nath et al, (2012) [36], 16 species (Ganesh and Mouli, et al.,2007) [37]. There are many more species are remains and yet to be explored.
Very recently, Sathe and Bhoje (2014) [38] reported amphibians of economic importance for use in biological control of insect pests. However, very little attention is paid on the diversity, conservation, protection and utility of amphibians in India. Keeping in view all above facts, present work was carried out. In this study, a list of amphibians and their diversity in and around wetlands of the study area are carried out. Apart from that the feeding habits and food utilization of amphibians are also evaluated to find out the effect of the role of biological control in an ecosystem. No study has been initiated so far about the amphibians in the study area. Hence the present study was planned to conduct with it the objectives of to document the diversity of different species of amphibians present in the Cauvery delta region, know the habitat utilization, document the biological control system of insect pest in cultivated fields and other ecosystem services of amphibian fauna and recommend suitable strategies for the conservation of amphibian fauna in the study area.
Materials and Methods
Cauvery Delta Zone (CDZ) lies in the eastern part of Tamil Nadu between 10.00o-11.30o North and between 78.15o–79.45o East. It is bounded by the Bay of Bengal on the East and the Palk straight on the South, Trichy district on the west, Perambalur, Ariyalur districts on the north west, Cuddalore district on the North and Puddukkottai district on the South West. The present study of amphibians is intended to carryout in different habitats such as Aquatic, cultivated field, wood land, grasslands, etc. of Kuttalam Taluk of Nagapattinam Districts.
The survey was performed using the visual-encounter method (Heyer, et al., 1994) [39], at a weekly interval in all possible habitats and microhabitats such as open land, cultivated field, water bodies, bushy land, wood lands mainly during the rainy seasons. Places such as grassy areas, leaf litter, logs, along bodies of water, rock crevices, vegetation, road edges was thoroughly searched for detecting amphibian species. The timing of the survey was between 6.00 pm and 11.00 pm in night and 5.30 am and 8.00 am in early morning. The Road transect method was applied for the estimation of abundance of amphibians. All Anurans species were recorded by direct sighting method and also by recording the calls from the concerned species. No live specimen was collected from the study area during the study period. Photographs of the sighted animals were taken by using camera for documentation and identification purpose. The identification was confirmed by using various diagnostic keys and publications (Das & Dutta, et al., (1998); Chanda, et al., (2002); Daniel, et al., (2002); Daniels, et al., (2005)) [40- 43]. Also some identification was confirmed by consulting herpetologists.
Species diversity index (H¯) was determined by Shannon Wiener’s index (Shannon & Weaver,et al., 1949) [44]. H¯=−Σ pi ln pi, where, pi=ni/N, which denotes the importance probability of each species in a population; ni =importance value for each species; N=total of importance value. Concentration of dominance (Cd), known as Simpson index, was measured according to Simpson (1949): Index of dominance (Cd)=Σ (ni/N)2. Species richness or variety index (d) is the mean number of species per sample and determined using the formula of Margalef (1958) [45].
Dead or road killed specimens of amphibians were collected for examine the gut content to know the food remains. The foods were separated item wise by incising the stomachs longitudinally and preserved in preservatives. The qualitative analysis of food items was made. The stomach contents of each frog were categorized into groups: (a) animal foods and (b) accidental food particles. The quality of consumed food by the frog species was also made. Food was initially categorized in large groups, mainly phylum and class. Food was analyzed using two parameters: the frequency of occurrence of different food groups, and the degree of food “preference” for each prey item. The frequency of occurrence was defined as the percentage of stomachs of each frog species containing a particular type of food. A food group was then classified as constant when registered in >50% of the stomachs of a particular species, secondary when present in 25%-50% of stomachs, or accidental when observed in <25% of the stomachs (DAJOZ, et al., 1983) [46]. Later the insect food items were identified at order level by consulting books, guides and specialists of the respective fields. Identification was done by consulting the books (Borror and Delong, et al., 1954 and Imams, et al., 1965) [47, 48]. Stomach content of individual frog was measured for quantitative analysis according to Hartley (1948). The frequency of every food item was transformed into percent frequency of occurrence in two ways: firstly, calculation was made in relation to total number of food items found in the total number of stomachs and secondly, in relation to the total amount of food contents found in the stomachs studied.
Results
Diversity of amphibians
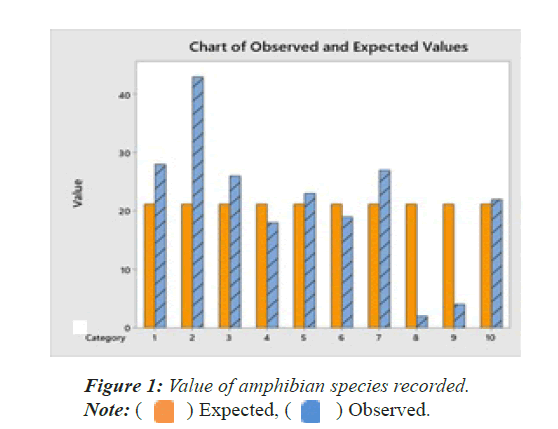
A total of 212 individuals of amphibians belonging to 10 species, 7 genera and 4 families were recorded from different parts of the study area as mentioned below (Table 1). The detailed species accounts of these amphibian species along with their scientific names, common names, their status were given (Plate 1). This forms the first scientific document of the amphibians of the study area. The chi-square test of amphibians recorded in the study area shows that there is significant differences among the species X2=59.5094, df=9, p=0.000. All the 10 species recorded are in the status of Least Concern by IUCN. The observed and expected values are given in Figure 1 and Table 1.
| S. No | Family | Common name | Species name | Number | Chi-square | IUCN category |
|---|---|---|---|---|---|---|
| 1 | Bufonidae | Common indian toad | Duttaphrynus melanstictus | 28 | 2.1811 | LC |
| 2 | Dicroglossidae | Common skittering frog | Euphlyctis cyanophlyctis | 43 | 22.417 | LC |
| 3 | Six-toad frog | Euphlyctis hexadactylus | 26 | 1.0868 | LC | |
| 4 | Indian bull frog | Hoplobatrachus tigerinus | 18 | 0.483 | LC | |
| 5 | Jerdon’s bull frog | Hoplobatracus crassus | 23 | 0.1528 | LC | |
| 6 | Rice field frog or asian grass frog | Fejervarya limnocharis | 19 | 0.2283 | LC | |
| 7 | Kerala warty frog | Fejervarya keralansis | 27 | 1.5868 | LC | |
| 8 | Microhylidae | Ornate narrow-mouthed frog | Microhyla ornate | 2 | 17.3887 | LC |
| 9 | White-bellied pug-snout frog | Ramanella variegata | 4 | 13.9547 | LC | |
| 10 | Rhacophoridae | Common Indian tree frog | Polypedates maculates | 22 | 0.0302 | LC |
| Total | 212 |
LC: Least Concern
A total of 212 individuals of amphibians sighted shows the diversity indices of the species were analysed and Dominance (D) of 0.1281 with Lower limit of 0.1215 and upper limit of 0.1466. Similarly the Simpson indices (1-D) of 0.879 with a Lower limit of 0.8533 and upper limit of 0.8784. The Shannon index shows that 2.131 with a lower limit of 2.05 and upper limit of 2.17. Similarly the Evenness (eH/S) shows 0.8426 with a lower limit of 0.8426 with a lower limit of 0.777 and upper limit of 0.8763 (Table 2).
| Diversity indices | Lower | Upper | |
|---|---|---|---|
| Taxa_S | 10 | 10 | 10 |
| Individuals | 212 | 212 | 212 |
| Dominance_D | 0.1281 | 0.1215 | 0.1466 |
| Simpson_1-D | 0.8719 | 0.8533 | 0.8784 |
| Shannon_H | 2.131 | 2.05 | 2.17 |
| Evenness_eH/S | 0.8426 | 0.777 | 0.8763 |
Habitat preferences
The results of habitat preferences of amphibians shows that mostly the frogs and toads prefer Aquatic, Agricultural lands and terrestrial ecosystems for their life support. Few species seen only in cultivated field whereas some are seen only in the terrestrial habitats especially the woodlands (Table 3).
| Sl. No | Family | Common name | Species name | Habitat preferences |
|---|---|---|---|---|
| 1 | Bufonidae | Common indian toad | Duttaphrynusmelanstictus | Agri, Land, Terrestrial |
| 2 | Dicroglossidae | Common skittering frog | Euphlyctis cyanophlyctis | Aquatic, Agri. Land |
| 3 | Six-toad frog | Euphlyctis hexadactylus | Aquatic, Agri, Land, Terrestrial | |
| 4 | Indian bull frog | Hoplobatrachus tigerinus | Aquatic, Agri, Land, Terrestrial | |
| 5 | Jerdon’s bull frog | Hoplobatracus crassus | Aquatic, Agri, Land, Terrestrial | |
| 6 | Rice field frog or asian grass frog | Fejervarya limnocharis | Agri, Land | |
| 7 | Kerala warty frog | Fejervarya keralansis | Agri, Land, Terrestrial | |
| 8 | Microhylidae | Ornate narrow-mouthed frog | Microhyla ornate | Terrestrial |
| 9 | White-bellied pug-snout frog | Ramanella variegate | Aquatic, Terrestrial | |
| 10 | Rhacophoridae | Common indian tree frog | Polypedates maculates | Agri, Land, Habitations |
Food preferences
Qualitative analysis: A total of 13 food items was identified in the 25 stomach contents of Indian Bull frog (Hoplobatrachus tigerinus). The frog has been identified as omnivore because all the food items found in the stomach contents were aquatic, semi-aquatic and terrestrial animals and plants. The major food items were insects (56.93%), Crustacians (12.18%), Fishes (8.23%), Arachnids (11.01%), annelids (5.8%), amphibians (2.24%) and plant maters (3.61%) (Figure 2).
Similarly 20 numbers of dead carcasses of Common Indian Toad (Duttaphrynus melanosticus) were dissected for gut content analysis. The toad seems to be the carnivores fed mainly on insects (82.78%), arachnids (12.82%), annelids (2.87%) and Diplopods (millipeds) (1.53%) (Figure 3).
Among the insect food items recovered from the stomach of Hoplobatrachus tigerinus shows it consists higher percentage of Orthopteran insects (34.75%) followed by Hymenoptera (23.98%), Isoptera (17.36%), Coleoptera (12.23%) and miscellaneous species of insects (11.68%) respectively (Figure 3). Similarly in the case of Duttaphrynus melanosticus the variations of food intake of insects are Hymenoptera (23.98% followed by Orthoptera (28.92%), Isoptera (21.68%), Coleoptera (14.87%) and miscellaneous species of insects are (3.29%) respectively (Figure 4).
Among the 25 specimens of Hoplobatrachus tigerinus analyzed, the snout-vent length (SVL) varied from 14.11 mm to 47.98 mm (females=46.03 ± 2.49; males=43.13 ± 7.37; juveniles=20.73 ± 3.59). Neither the number of prey items (R2=0.0; F1, 25=0.0; P=0.97556), nor the total volume (R2=0.04; F1, 25=1.19; P=0.28636) were significantly related to size (SVL). The same pattern was observed in the results of related jaw with volume (R2=0.03; F1, 25=0.26; P=0.61) and number of prey items (R2=0.02; F1, 25=0.51; P=0.47) (Figures 5a-5j).
Dicussion
The present study reveals that the study area holds handful diversity of amphibian fauna. Species richness is simply the number of species in a fauna, while equitability represents some measure of the evenness of their distribution. In this study high value of dominance index compare to species diversity of amphibians indicates the lower diversity and may lead to lower stability of the community (MacArthur, et al., 1955) [49]. High abundance of D. melanostictus compared to other species which may lead to the lower stability in this community. D. melanostictus is cosmopolitan in distribution (Dutta, et al., 1997) [50] and is known to occur in a variety of habitats, especially in disturbed areas (Inger, et al., 1984) [51]. Species with the broadest habitat distribution should show high levels of plasticity. Daniels (1992) stated that the number of individuals that represents each species in community may vary from place to place depending on the amount of rainfall, available habitats and human interference as the structure and diversity of an amphibian community is determined by the availability of food, moisture and micro habitat. Significantly amphibians were encountered in leaf litters, as leaf litters may provide a wider range of microhabitats, allowing more individuals and more species to coexist in the litter microhabitat (Fauth, et al., 1989) [52]. Furthermore, Fauth found that species richness increased rapidly with an increase in leaf litter depth, as did herpe to faunal density. Deeper leaf litter may provide a wider range of micro-habitats, allowing more individuals and more species to coexist in the litter micro habit. All the frogs and toads are insect eating with few exceptions.
Many habitat types may occur within an area, amphibians may utilize only a few of these. The number of individuals that represents each species in community may vary from place to place depending on the amount of rainfall, available habitats and human interference as the structure and diversity of an amphibian community is determined by the availability of food, macro and micro habitat (Daniels & Ishwar, et al 1994) [53].The habitat of the study areas were vastly cultivated with paddy fields (Laxmi, et al., 2011) these kind of ecosystems well attracted to amphibian species may use of various purpose such as food (insects) and home grounds etc. Amphibians important to agriculturalists, they take play a key role in ecosystem functioning and act as predator, mainly as consumers of insect pest (Duellman & Trueb, et al., 1986) [54]. In the present study it is identified variety of amphibian species utilizing four different habitats namely Agricultural land, Aquatic, habitations and terrestrial habitats.
In the present study Hoplobatrachus tigerinus mainly feed on animal and plant matters as food where as Duttaphrynus melanosticus feeds on only animal matters especially insect pests. Schoener's (1974) review, found that habitat, food, and time (in that order) were the most important niche dimensions in most community studies. Here in this study it was analyzed one of the dimension, therefore concrete conclusion can’t be made on the niche overlap or coexist of these amphibian species in the same biota. Furthermore, Niche metrics have been used to infer the role of competition, but the interpretations are not straight forward (Colwell & Futuyma, et al., 1971) [55] a small overlap may indicate that competition is not important, but may also result from intense competition. Theoretically, two niches may overlap 100% on some resource axes, as long as they are separate on others (McNaughton & Wolf, et al., 1979) [56]. Niche theory holds that two coexisting species will tend to reduce overlap in use of limited resources to avoid competition (MacArthur & Levins, et al., 1967) [57]. The theory of community ecology predicts that spatial and temporal environmental variations have a crucial role in species coexistence (Desbiez, et al., 2009) [58]. However, the present study on amphibian community is just a model to show the microhabitat occupancy by the amphibians in the human settlements and competition among them as, spatial resource partitioning may be one of the chief indicators of interspecific interactions.
The results indicate that the dominant food items of Hoplobatrachus tigerinus are insects, which made up almost all of its dietary composition. From this, ants from family Formicidae, followed by termites from family Termitoidae, and lastly, beetles from order Coleoptera. Toads from the family Bufonidae are generally nondiscriminatory predators. They eat every moving organism of appropriate size that happened to be in their foraging range (Hirai and Matsui, et al., 2002). From the same study of Hirai and Matsui (2002), quantitatively the dominant stomach content of juvenile toads (Bufo japonicus) is ants, whereas adult toads prefer insects from the order Coleoptera, followed by ants and diplopods. The results from this study further support this statement, as the major diet of Duttaphrynus melanosticus are ants and termites. This strong tendency towards ants and termites indicates that Duttaphrynus melanosticus is an "ant specialist". In order to gain enough energy, Duttaphrynus melanosticus feeds on a large amount of ants and termites to compensate for the small prey size. This is similar to the findings of Santars and Junca (2007) on Bufo grarlulosus, a toad also from the family Bufonidae. In their study, they found that the frequency of occurrence of termites (order Blattodea, family Termitoidae) in the diet of Bufo granulosus and Formicidae presented the largest occurrence frequency at almost 100%. Sand grains are commonly found in the stomach of Hoplobatrachus tigerinus and Duttaphrynus amelanosticus. Although the most immediate answer to the occurrence of sand is accidental ingestion during prey capturing, the fine grains of sand may help in the elimination of intestinal parasites, and provide roughage to assist in grinding up of arthropod exoskeletons (Santos, et al., 2004).
Duttaphrynus melanosticus from this study showed an obvious Iiking to insectivorous diet, The dominant prey items are Formicidae which took the dietary composition, followed by Termitoidae, Coleoptera and other unidentified Hymenoptera. Dietary composition as observed in this study was similar to those reported by Ahmad and Ahmad (2009) and, Nurul and Ibrahim (2008) for Duttaphrynus melanosticus from the same study site. Even though Duttaphrynus melanosticus is known to live in various habitats such as forest floor, stream side, and pool, the main dietary components of Duttaphrynus melanosticus are terrestrial arthropods. This correlates with the study of Sole et al., (2009) that stated, terrestrial invertebrates usually dominate the diet of most anurans even in those aquatic or semi aquatic species. The high frequency of occurrence of ants and termites in the diet of Duttaphrynus melanosticus indicates that it is indeed an "ant specialist". The plant material and other indigestible items found in many stomachs of Duttaphrynus melanosticus was most probably ingested unintentionally together with rapidly swallowed animal prey, as described for other anurans (Dietl Sol., 2009, Sole, et al., 2009). Duttaphrynus melanosticus is observed to be a clumsy eater that more often than not, accidentally gobbled down other indigestible items while capturing prey items.
Conclusion
Generally the amphibians are aquatic and terrestrial inhabitant in which aquatic is more important in their life span for feeding, Breeding and most importantly for metamorphosis tadpoles. Remaining habitats are lack of water source and microhabitat also alteration of habitat or cleaning are the major reason for less population of amphibians in this field. There were changing habitat and climates are regulating the population structure inhabitant location also. This study obtained the anuran population are more preferable in aquatic habitat of pond and cultivated habitat. Due to habitat loss, fragmentation and urbanization, a vast land area that provide roost resource for amphibians starts depleting at a greater rate. Hence study on the diversity and habitat is a need of the hour in order to make conservation priorities. This study generated a base line data on the amphibian fauna of this region, which may help in further studies.
IUCN considers most of the amphibian species as Critically Endangered due to its previously known restricted distribution and it was considered as one of the Lost Amphibians of India. In this investigation, it is clear that a long-term study in this area is needed on the ecology and distribution of herpetofauna to learn the wealth of this virgin ecosystem and there are possibilities to recover/rediscover/ occurrence of new species.
Dietary information is pivotal for successful development of conservation strategies on species level and the understanding of ecosystem function. Unfortunately, this kind of information is not available for the vast majority of taxa and is often incomplete. Seasonal variations, ontogenetic shifts, and relationships between site-specific prey availability, presence of potential competitors, and diet composition are commonly not addressed. Future studies should focus on these issues. In the light of global amphibian declines an Amphibian Conservation Action Plan (ACAP) was formulated by the International Union for conservation of nature to prevent further biodiversity loss and captive breeding programmes are being developed for the most threatened species. Such approaches depend crucially on autecological knowledge such as information on the diet of the target species. Here, every kind of information can be valuable. Environmental conditions and habitat of species play an important role in the distribution and diversity of species. Hence this survey provides baseline data and scientific information for conservation of amphibians from the study area. All the above findings it is understood that the amphibians play an important role for Insect pest control and act as agent of Biocontrol. Possible recommendations to save Amphibians:
1. Captive breeding programmes for endangered species can be done in situ.
2. Reintroduction programs place amphibians back into wild habitats in the hope that new populations can be established.
3. Introduced species are being removed where they threaten native species.
4. Measures taken to protect amphibian habitats.
5. Land and water management techniques modified to minimize the impact on amphibians.
6. Restoration of habitats and natural processes.
7. Preparing and implementing species recovery programmes for selected species.
8. Eat organic food by reducing pesticide and fertilizer use, you directly help in reducing the amount of chemical contamination that affects many amphibian species.
9. Avoid releasing environmental estrogens into the water. Environmental estrogens are known to affect amphibian worldwide including human being.
10. Pesticides kill amphibians and insects that amphibians eat hence their use should be avoided.
11. Leave natural and artificial ground cover (e.g. old wood cover boards or dead wood) in your backyard. Ground cover provides moist shelter to amphibians.
12. Leave native aquatic vegetation growing at your pond. It provides food, refuge and breeding habitat for amphibians.
13. Join campaigns to stop frog and salamander trade. Frog trade has been responsible for introducing amphibian diseases and non-native predators.
14. Protect amphibian from pets. Cats and dogs can disturb breeding activities of frogs and salamanders. Be a responsible pet owner and discourage your pets from disturbing amphibians.
References
- Bukhary, A., Khair, A. (2009). Stomach Content Analysis of A Few Species of Frogs from Gunung Inas Forest Reserve, Kulim Kedah (In Malay). Unpublished Bachelor of Science dissertation. University Sains Malaysia, Penang.
- Anil, Z., Dinesh, KP., Radhakrishnan, C., Kunhikrishnan, E., Palot, MJ., Vishnudas, C.K. (2011b). A new species of Polypedates tschudi (Amphibia: Anura: Rhacophoridae) from southern Western Ghats, Kerala, India. Biosystematica., 5(1): 49-53.
- Biju, SD., Shouche, Y., Dubois, A., Dutta, SK., Bossuyt, F. (2010). A ground-dwelling rhacophorid frog from the highest mountain peak of the Western Ghats of India. Curr. Sci., 98(8): 1119–1125.
- Biju, SD., Bossuyt, F. (2003). New frog family from India reveals an ancient biogeographical link with the Seychelles. Nature., 425:711-714.
- Biju, SD., Bocxlaer, IV., Giri, VB., Loader, SP., Bossyut, F. (2009). Two new endemic genera and a new species of toad (Anura:Bufonidae) from the Western Ghats of India. BMC Res. Notes., 2: 241.
- Biju, SD., Bocxlaer, IV., Mahony, S., Dinesh, KP., Radhakrishnan, C., Anil, Z., Giri,V., Bossuyt, F. (2011). A taxonomic review of the Night frog genus Nyctibatrachus Boulenger, 1982 in the Western Ghats, India (Anura: Nyctibatrachidae) with description of twelve new species. Zootaxa., 3029: 1-96.
- Blaustein, AR., Wake, DB. (1990). Declining amphibian populations–a global phenomenon? Trends in Ecology & Evolution., 5: 203 – 204.
- Borror, DJ., Delong, DM. (1954). An Introduction to the Study of Insects. Holt Rinehart and Winston, United States of America, Library of Congress. 1030 + ix pp.
- Bridges, CM. (1997). Tadpole swimming performance and activity affected by acute exposure to sub-lethal levels of carbaryl. Environmental Toxicology and Chemistry., 16:1935–1939.
- Bridges, CM., Semlitsch, RD. (2000). Variation in pesticide tolerance of tadpoles among and within species of Ranidae and patterns of amphibian decline. Conservation Biology., 14:1490–1499.
- Chanda, SK. (2002). Handbook–Indian Amphibians. Zoological Survey of India, Calcutta, India.
- Clarke, BT. (1997). The natural history of amphibian skin secretions, their normal functioning and potential medical applications. Biol. Rev.,72:365-379.
- Colwell, RK., Futuyma, DJ. (1971). On the measurement of niche breadth and overlap. Ecology., 52:567-576.
- Cushman, SA. (2006). Effects of habitat loss and fragmentation on amphibians: a review and prospectus. BiolConservat., 128:231–240.
- DAJOZ, WE. (1983). Ecologia Geral. São Paulo, Vozes. 474 p.
- Daniel, JC. (2002). The Book of Indian Reptiles and Amphibians. Bombay Natural History Society and Oxford University Press., 238 pp.
- Daniels, RJR. (2005). Amphibians of Peninsular India. Universities Press, Hyderabad, India: 286. Das, I., 2002. A Photographic Guide to Snakes and other Reptiles of India. New Holland publications, London, UK: 144 pp.
- Daniels, RJ. (1992). Geographical distribution patterns of amphibians in the Western Ghats, India. Journal of Biogeography., 19: 521-529.
- Daniels, RJ., Ishwar, NM. (1994). Rarity & the herpetofauna of the southern Eastern Ghats, India. Cobra., 16: 2–14.
- Das, I., Dutta, SK. (1998). Checklist of amphibians of India with English and common names. Hamadryad., 23: 63–68.
- Daszak, P., Strieby, A., Cunningham, AA., Longcore, JE., Brown, CC., Porter, D. (2004). Experimental evidence that the bull frog (Rana catesbeiana) is a potential carrier of chytridiomycosis, an emerging fungal disease of amphibians. Herpetol. J., 14:201-207.
- Demaynadier, PG., Hunter, Jr. (1998). Effects of silvicultural edges on the distribution and abundance of amphibians in Marine. Conservation Biology., 12:340–352.
- Desbiez, J., Santos, S., Keuroghlian, A., Bodmer, RE. (2009). Niche partitioning among White-lipped Peccaries (Tayassu pecari), Col lared Peccaries (Pecari tajacu), and feral pigs (Sus scrofa). Journal of Mammalogy., 90 (1):119–128.
- Dietl, J., Engels, W., and Sole, M. (2009). Diet and feeding behaviour of the leaf-litter frog Ischnocnerna hen selii (Anura: Brachycephalidae) in Araucaria rain forests on the Serra Geral of Rio Grande do Sul, Brazul. Journal of Natural History., 43 (23-24):1473 -1 483.
- Dinesh, K.P., Radhakrishnan, C. (2009). Amphibia.Zool.Surv. India. Fauna of Tamil Nadu, State Fauna Series., 17:165–187.
- Dinesh, K.P., Radhakrishnan, C., Bhatta., G. (2008). A new species of Nyctibatrachus Boulenger (Amphibia: Anura: Nyctibatrachidae) from the surroundings of Bhadra Wildlife Sanctuary, Western Ghats, India. Zootaxa., 1914: 45–56.
- Dinesh, K.P., Radhakrishnan, C., Gururaja, KV., Deuti, K., Bhatta, G. (2013). A Checklist of Amphibia of India with IUCN Red list Status. Zoological Survey of India.
- Dinesh, K.P., Radhakrishnan, C. (2011). Checklist of amphibians of Western Ghats. Frog leg., 16: 15-21.
- Dinesh. K.P, Radhakrishnan. C, Gururaja, K.V, Deuti, K., Bhatt, G.K. (2011). A checklist of amphibian of India.
- Dodd, C. K. Jr. (1996). Use of terrestrial habitats by amphibians in the sand hill uplands of north-central Florida. Alytes., 14:42–52.
- Duellman, W.E., Trueb, L. (1986). McGraw-Hill, New York. Duellman WE, Trueb L. Biology of amphibians. The John Hopkin University Press, Maryland, USA, Biology of Amphibians., 1994: 22- 28.
- Dutta, S.K. (1997). Amphibians of India and Sri Lanka (Check list & Bibliography). Odyssey Publ. House, Bhubaneshwar 342.
- Dutta, S.K. and Ray, P. (2000). Micro hylasholigari, a new species of micro hylid frog (Anura: Micro hylidae) from Karnataka, India. Hamadryad., 25(1): 38–44.
- Fauth, J.E., Crother, B.I., Slowinski, J.B. (1989). Elevational Patterns of Species Richness, Evenness, and Abundance of the Costa Rican Leaf-Litter Herpetofauna. Biotropica 21(2): 178-185.
- Findlay, C.S., Houlahan, P. (1997). Anthropogenic correlates of species richness in southeastern Ontario wetlands. Conservation Biology., 11 (4) : 1000-1009.
- Freemark, K., Boutin, C., (1995). Impacts of agricultural herbicide use on terrestrial wildlife in temperate landscapes: a review with special reference to North America. Agriculture, Ecosystems and Environment., 52:67–91.
- Frost, D.R. (2013). Amphibian Species of the World: an Online Reference. Version 5.6 Electronic Database accessible at American Museum of Natural History, New York, USA.
- Ganesh, S.R., Chandramouli, S.R. (2007). A Study of the herpetofaunal community in Mannampandal, Nagapatinam District, Tamil Nadu. Cobra., 1(4): 33–43.
- Gibbs, J.P. (1998). Distribution of woodland amphibians along a forest fragmentation gradient. Landscape Ecology., 13:263–268.
- Gray, M.J. (2002). Effect of anthropogenic disturbance and landscape structure on body size, demographics, and chaotic dynamics of Southern High Plains amphibians. Dissertation. Texas Tech University, Lubbock.
- Gray, M. J., Smith, L.M. (2004). Influence of land use on postmetamorphic body size of playa lake amphibians. Journal of Wildlife Management., 68: in press.
- Gururaja, K.V., Dinesh, K.P., Palot, M.J., Radhakrishnan, C., Ramachandra. T.V. (2007). A new species of Philautus Gistel (Amphibia: Anura: Rhacophoridae) from southern Western Ghats, India. Zootaxa., 1621(1): 1– 16.
- Hanson, GC., Groffman, PM., Gold, AJ.(1994). Symptoms of nitrogen saturation in a riparian wetland. Ecological Applications., 4:750– 756.
- Hartley, PHT. (1948). The assessment of the food of birds. J. Ibis., 90: 361 - 381.
- Herbeck, LA., Larsen, DR. (1999). Plethodontid salamander response to silvicultural practices in Missouri Ozark forests. Conservation Biology., 13:623–632.
- Heyer, WR., Donnelly, AM, Diarmid, RWM., Hayek, LC., Foster, MS. (1994). Measuring and monitoring biological diversity: Standard methods for Amphibians. Smithsonian Institution Press, Washington, DC.
- Hirai, T., Matsui, M. (2002). Feeding ecology of Bufo japorticus formosus from the montane region of Kyoto, Japan. Journal of Herpetology., 36(4) :719-723.
- Houlahan, J. E., Findlay, CS., Schmidt, BR., Meyers, AH., Kuzmin, SL. (2000). Quantitative evidence for global amphibian population declines. Nature., 404:752–755.
- Imams, AD. (1965). A General Textbook of Entomology. 9th Edition. Revised by Richards, O. W. and devies, R. G. Butler and Tanner. London. 886.
- Inger, R.F., Shaffer, HB., Koshy, M., Badke, R. (1984). A report on the collection of amphibians and reptiles from the Ponmudi, Kerala, South India. Journal of Bombay natural History Society., 81:406-427; 551-570.
- Joshy, S.H., Alam, M.S., Kurabayashi, A., Sumida, M., Kuramot, M. (2009). Two new species of the genus Euphlyctis (Anura, Ranidae) from southwestern India, revealed by molecular and morphological comparisons.
- Kanaujia, A., Kumar, A. (2013). Amphibians of Uttar Pradesh and their ecological importance. International Day for Biological Diversity, Water and Biodiversity., 148-154.
- Karthik, P. (2017). Status of the herpetofauna in the Cauvery Delta Region, Mannampandal, Tamil Nadu, India. IRCF Reptiles & Amphibians., 24(3): 180-186.
- Karunakaran, K., Jeevanandham, P. (2017). Amphibian diversity in different habitat of Agro ecosystem in Nagapattinam District. Int. J. Modn. Res. Revs., 5(4): 1539-1543.
- Laxmi, NB., (2011). Population of House Sparrow (Passer domesticus) in Yellampet, Nizamabad District, Andhra Pradesh, India. Newsletter for Birdwatchers., 51(2): 21-22.
- Luo, H.R., Smith, L.M., Allen, B.L., Haukos, D.A. (1997). Effects of sedimentation on playa wetland volume. Ecological Applications., 7:247–252.
- MacArthur, R. (1955). Fluctuations of Animal Popula tions and a Measure of Community Stability. Ecology., 36 (3): 533-536.
- MacArthur, R.H., Levins, R. (1967). The limiting similarity, convergence, and divergence of coexisting species. American Naturalist., 101:377-385.
- Margalef, R. (1958). Information theory in ecology. General Systematics., 3: 36–71.
- Marsh, D.M., Trenham, P.C. (2001). Metapopulation dynamics and amphibian conservation. Conservation Biology., 15:40-49.
- Mazzoni, R., Cunningham, A.A., Daszak, P., Apolo, A., Perdomo, E., Speranza, G. (2003). Emerging Pathogen of Wild Amphibians in Frogs (Rana catesbeiana) Farmed for International Trade. Emerg. Infect., 9(8): 995-998.
- McNaughton, S.J., Wolf, L.L. (1979). General Ecology. 2nd ed. Holt, Rinehart and Winston, New York, NY. Magura, T., Tóthmérész, B., Molnár, T. (2004). Changes in carabid beetle assemblages along an urbanization gra dient in the city of Debrecen, Hungary. Landscape Ecology., 19: 747–759.
- McWilliams, DA. (2008). Nutrition Recommendations for some Captive Amphibian Species (Anura and Caudata). The Canadian Association of Zoos and Aquariums Nutrition Advisory and Research Group (GARN-NARG).
- Nath. A., Sutradhar. S., Kalaimani, A., Vijayan, V., Kumar, K., Narayana, L., Naresh, B., Babuurao, G., Dharwadkar, S., Krishnan, G., Vinoth, B., Maniraj, R., Reddy, D.M., Mallaiah, D.A., Swamy, K. (2012). Herpetofaunal assemblage with special emphasis on community structure and spatiality in amphibians of Cauvery delta region, Tamil Nadu. Asian J. Conserv. Biol., 1(2): 78-85.
- Naughton, G. P., Henderson, C.B., Foresman, K.R., McGraw, R.L. (2000). Long-toed salamanders in harvested and intact Douglas-fir forests of western Montana. Ecological Applications., 10:1681–1689.
- Dalila, N., Jaafar, I. (2008). Stomach Content Analysis of 3 Species of Lowland Dipterocarp Forest Frogs in Malaysia: Proceedings of the Sixth Regional IMT-GT Uninet Conference 2008. University Sains Malaysia & IMTGT., 435 - 438.
- Relyea, R.A., Mills, N. (2001). Predator-induced stress makes the pesticide carbaryl more deadly to gray tree frog tadpoles (Hyla versicolor). Proceedings of the National Academy of Sciences of the United States of America., 98:2491–2496.
- Santos, E.M., Almeida, A.V., Vasconcelo's, S.D. (2004). Feeding habits of six anuran (Amphibia: Anura) species in a rainforest fragment in Northeastern Brazil. Iheringia, Serie Zoologia, Porto Alegre., 94(4): 433- 438.
- Sathe, T.V. Bhoje, P.M. (2014). Amphibians for insect pest management. Recent Trends in biological control., 22: 160-171.
- Schad, K. (2008). Amphibian Population Management Guidelines. Amphibian Ark Amphibian Population Management Workshop San Diego, CA, USA. Amphibian Ark., 8: 31-34.
- Schoener, T.W. (1974). Resource partitioning in ecological communities. Science., 185(4145): 27-39.Semlitsch, R.D. (2000). Principles for management of aquatic-breeding amphibians. Journal of Wildlife Management., 64(3):615–631.
- Shannon, C.E., Weaver, W. (1949). The mathematical theory of communication, University of Illinois press, Urbana. Simpson, E. M. 1949. Measurement of diversity. Nature., 163:688.
- Simpson, E.M. (1949). Measurement of diversity. Nature., 163:688.
 ) Expected, (
) Expected, ( ) Observed.
) Observed. 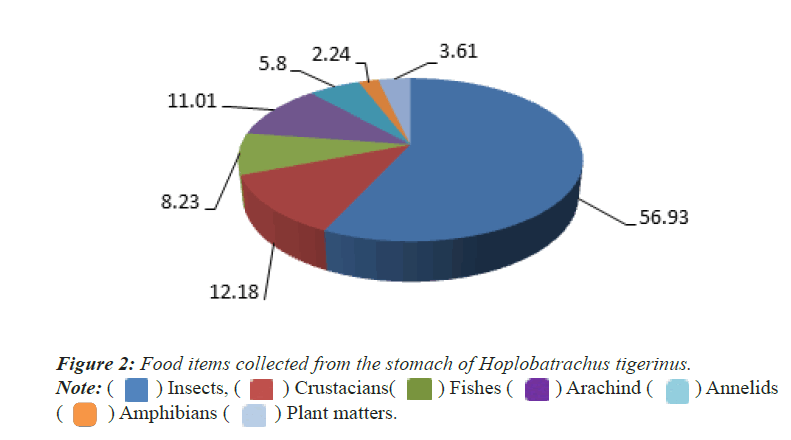
 ) Insects, (
) Insects, ( ) Crustacians(
) Crustacians( ) Fishes (
) Fishes ( ) Arachind (
) Arachind ( ) Annelids
(
) Annelids
( ) Amphibians (
) Amphibians ( ) Plant matters.
) Plant matters. 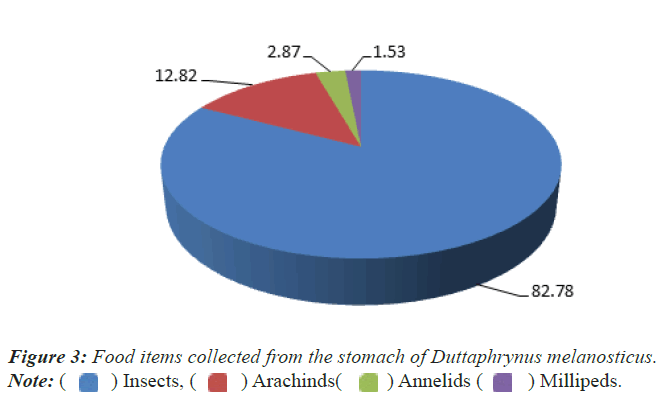
 ) Insects, (
) Insects, ( ) Arachinds(
) Arachinds( ) Annelids (
) Annelids ( ) Millipeds.
) Millipeds. 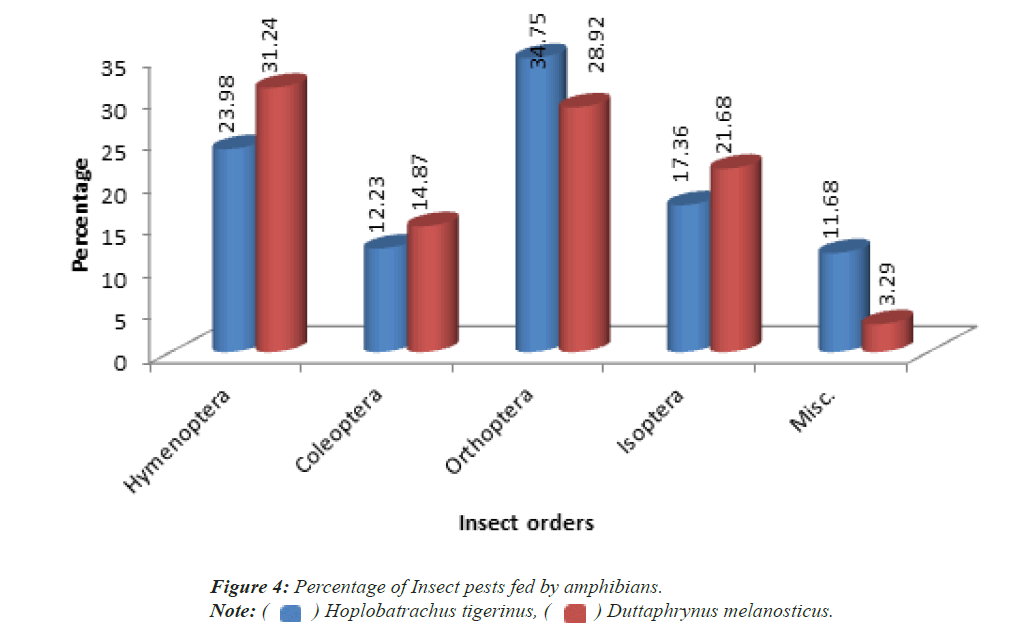
 ) Hoplobatrachus tigerinus, (
) Hoplobatrachus tigerinus, ( ) Duttaphrynus melanosticus.
) Duttaphrynus melanosticus. 