Research Paper - Journal of Clinical Ophthalmology (2019) Volume 3, Issue 3
Dissociation between the onset times of convergence eye movements and pupillo-constrictions in the near response of humans.
Haruo Toda1*, Atsuhiko Iijima2, Isao Hasegawa1,31Department of Physiology, Niigata University School of Medicine, Niigata, Japan
2Department of Bio-cybernetics, Faculty of Engineering, Niigata University, Niigata, Japan
3Center for Transdisciplinary Research, Niigata University, Niigata, Japan
- Corresponding Author:
- Haruo Toda
Department of Orthoptics and Visual Sciences
Faculty of Medical Technology, Niigata University
of Health and Welfare, Shimami-machi 1398
Kita-ku, Niigata-shi, Niigata 950-3198, Japan
Tel: +81-25-257-4753
E-mail: toda@nuhw.ac.jp
Accepted date: December 10, 2019
Citation: Toda H, Iijima A, Hasegawa I. Dissociation between the onset times of convergence eye movements and pupillo-constrictions in the near response of humans. J Clin Ophthalmol. 2019;3(3):174-179.
Abstract
Objective: Among the triad of the near response, the cross-linkages between the convergence eyemovement-related system and the lens accommodation-related system are well known and the accommodative convergence to accommodation (AC/A) ratio is widely used in the diagnosis of strabismus. Conversely, the function of the cross-linkage between the pupillo-constriction-related system and the convergence eye movement-related system is not fully understood. To examine this issue, we utilized an anticipatory convergence paradigm.
Materials and Methods: Nine healthy adult males participated in the experiments. The positions and pupil diameters of both eyes were recorded using a pair of infrared eye cameras, where the participant looked at far and near light emitting diodes (LEDs) that alternately lit at the front of him in a regular interval (1 s) or random interval pattern.
Results: Under the regular interval conditions, convergence eye movements in advance of the onset of near LED (anticipatory convergence) were frequently observed. In contrast, pupillo-constrictions were observed after the onset of near LED even with the anticipatory convergences. This convergenceversus pupil discrepancy was found in all nine participants and the difference in the onset times between convergence eye movement and pupillo-constriction was significant.
Conclusions: Our data suggest the complexity of the near response system in humans. Further neuronal and clinical investigations are required to better understand this concept.
Keywords
Near response, Convergence eye movement, Pupillo-constriction, Anticipation.
Introduction
When frontal-eyed animals (e.g. primates and cats) visually follow an approaching target, three extra and intra-ocular muscular movements are elicited at the same time: convergence eye movement, lens accommodation, and pupillo-constriction [1,2]. This triad is known collectively as the near response or near reflex. Within the three components of the near response, a functional cross-linkage between the convergence eye movement and lens accommodation is well known [3,4], even in human infants [5]. In clinical applications, the accommodative convergence to accommodation (AC/A) ratio is widely used for the diagnosis of esotropia [6] and the adoption of convergence accommodation to convergence (CA/C) for the diagnosis of exotropia has been previously proposed [7]. On the other hand, the nature of functional crosslinkage between the pupillo-constriction and the other two components, especially convergence eye movement, remains controversial [8-12].
In this study, we recorded the positions and sizes of both pupils simultaneously in the phase of normal convergence eye movement and that of the anticipatory convergence (i.e., convergence eye movement prior to the onset of the near visual target) [13-15] and observed a discrepancy in the onset times of the anticipatory convergence and pupillo-constriction.
Materials and Methods
Subjects
Nine healthy volunteers of various ages (22-64 years, 100% male) (Table 1) participated in the recording sessions. One of the subjects wore scleral contact lenses because of myopia. Including him, all the subjects had a corrected or uncorrected visual acuity adequate for fixating upon the visual targets. Two subjects understood the underlying hypothesis of the experiment in detail, one subject was given limited information for the hypothesis and the others were not informed of the details. This study was approved by the Niigata University Ethical Committee.
| Subject | Age (years) | Random | Regular | ||||
|---|---|---|---|---|---|---|---|
| Sessions | Conv. | Pupil | Sessions | Conv. | Pupil | ||
| 1 | 22 | 3 | 144 ± 11 | 300 ± 19 | 7 | -229 ± 37 | 319 ± 7 |
| 2 | 22 | 3 | 178 ± 22 | 333 ± 33 | 6 | -233 ± 26 | 322 ± 11 |
| 3 | 22 | 3 | 167 ± 0 | 289 ± 29 | 7 | -200 ± 51 | 262 ± 29 |
| 4 | 24 | 2 | 217 ± 17 | 267 ± 0 | 2 | -267 ± 0 | 267 ± 0 |
| 5 | 35 | 2 | 183 ± 17 | 267 ± 33 | 4 | -356 ± 48 | 144 ± 29 |
| 6 | 42 | 2 | 150 ± 17 | 283 ± 83 | 2 | -133 ± 100 | 317 ± 17 |
| 7 | 44 | 2 | 100 ± 33 | 200 ± 67 | 10 | -437 ± 15 | 280 ± 9 |
| 8 | 58 | 3 | 189 ± 11 | 356 ± 106 | 6 | -139 ± 38 | 389 ± 7 |
| 9 | 64 | 2 | 167 ± 33 | 367 ± 33 | 3 | -311 ± 91 | 333 ± 19 |
Table 1. Mean onset times of the averaged convergence and pupillo-constriction during regular- and random- interval tasks. The mean onset times ± standard error of the means of the convergence eye movement (Conv.) and the left pupillo-constriction (Pupil) in the random- (Random) and the regular-interval (Regular) tasks are shown for each subject. Responses of the right pupil are omitted because there were no significant differences between the right and left pupillary responses. All units are presented in ms unless otherwise specified.
Visual stimulation and tasks
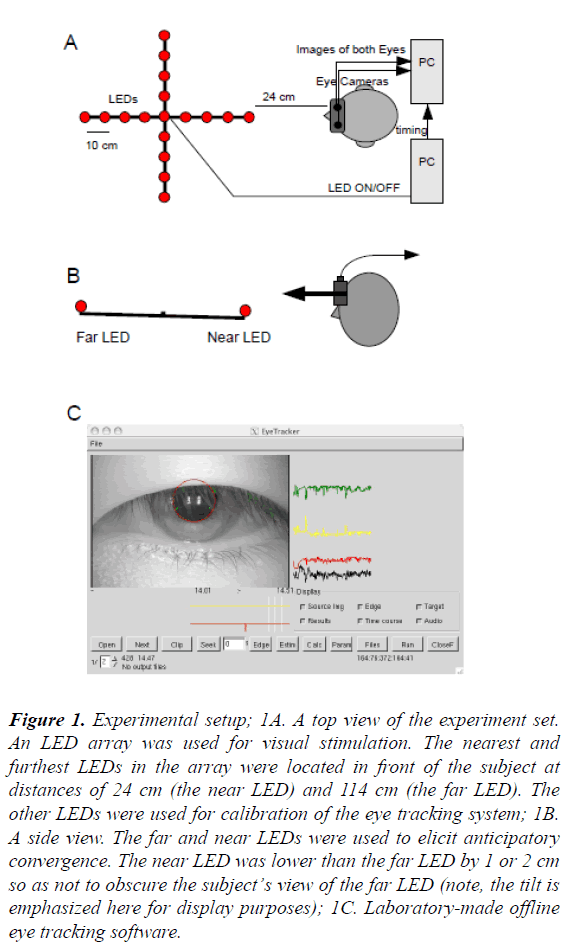
A light emitting diode (LED) array was used for visual stimulation. The LED array consisted of 25 red LEDs arranged in a cross formation. Each arm of the cross was 45 cm long and contained six LEDs (Figure 1A). Among the 25 LEDs, the far and near LEDs were used to elicit anticipatory convergence and the others were used for calibration of the eye tracking system. The nearest and furthest LEDs in the array were located in front of the subject at distances of 24 cm (the near LED) and 114 cm (the far LED), respectively. The near side of the bar was slightly lowered to ensure that both the far and near LEDs could be seen by the subject (Figure 1B). The status of all LEDs was controlled by a Turbo Pascal program running on a PC/AT compatible personal computer (Z-cosmos, Niigata, Japan) equipped with an outboard interval timer (ADC3134, CONTEC Co., Osaka, Japan) to ensure precise stimulation timing. The onset times of LEDs were also sent to the video eye tracking system for offline time alignment of the data.
Figure 1: Experimental setup; 1A. A top view of the experiment set. An LED array was used for visual stimulation. The nearest and furthest LEDs in the array were located in front of the subject at distances of 24 cm (the near LED) and 114 cm (the far LED). The other LEDs were used for calibration of the eye tracking system; 1B. A side view. The far and near LEDs were used to elicit anticipatory convergence. The near LED was lower than the far LED by 1 or 2 cm so as not to obscure the subject’s view of the far LED (note, the tilt is emphasized here for display purposes); 1C. Laboratory-made offline eye tracking software.
Three types of tasks were performed as follows as: a regularinterval task, a random-interval task, and an eye movement suppression task. During the regular-interval task, the far and near LEDs were lit, alternating at every other second. This paradigm is essentially the same as Kumar ’ s [14,15]. The subject was instructed to look at the newly lit LEDs as soon as possible. The random-interval task was similar in process to the regular-interval task except that the intervals between the onsets of the far and near LEDs were pseudo randomized in a range of 0.8 to 4 s. In the eye movement suppression task, the far and near LEDs were lit in an alternating fashion every other second but the subject was instructed to ignore them and keep looking forward. In each task, 49 (for the regular-interval task and eye movements suppression task) or 13 (for the randominterval task) trials were performed within approximately 100 s. The room was dimmed so that the unlit LEDs were invisible (<1 lux).
Recording system and data analysis
We used a pair of goggle-mounted infrared eye cameras (ET-60, Newopto, Kawasaki, Japan) to record images of both eyes. These eye-cameras were the see-through type, employing half-mirrors to reflect images of both eyes to cameras located at the top of the goggle. Therefore, the subject’s view was not obscured by the cameras. Images of both eyes were stored as MPEG2 files on a personal computer (Z-cosmos, Niigata, Japan) using a pair of video capture cards (GV-MVP/RX3, I.OData Co., Kanazawa, Japan) for offline analysis. The frame rate and spatial resolution of the video system were 30 fps, and 720 × 480 pixels for each eye.
The recorded MPEG2 files were analyzed by using laboratorymade video eye tracking software written in Object Pascal Lazarus, to detect the two-dimensional positions and diameter of the pupil using Hough transformation approach (Figure 1C). Then, the respective onset times of the convergence eye movements and pupillo-constrictions were sought in each trial with a laboratory-made software program written in REALbasic (https://xojo.com/products/).
At first, the software scanned the entire trial data to seek the point of the maximum gradient by fitting regression lines to long (297 ms) segments of the trace. When the time of the maximum gradient was found, the software began to scan backward, fitting regression lines to short (99 ms) segments of the trace repeatedly to seek the point at which the gradient of the trace exceeded a certain threshold of the first time. The threshold of 0.5 pixels per frame was used for both the convergence and the pupil traces.
Results
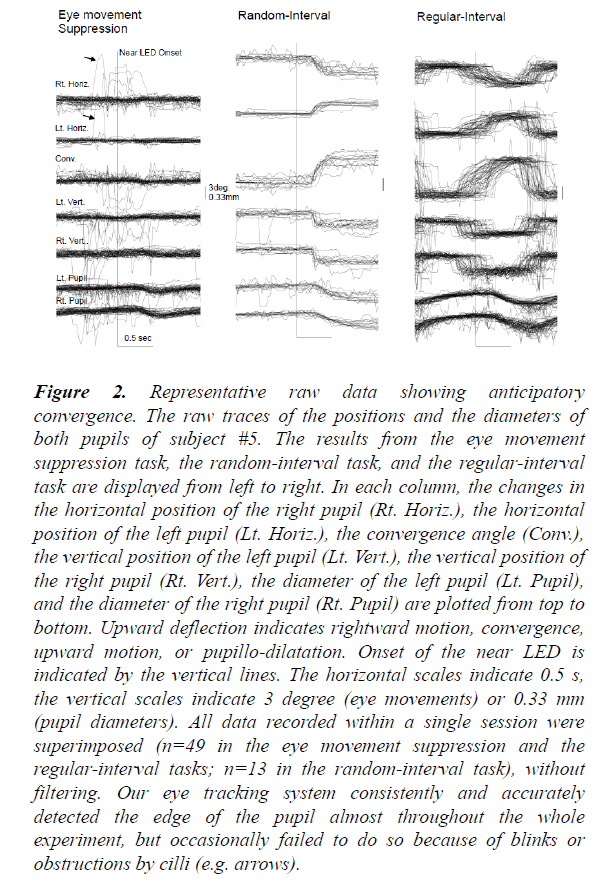
In Figure 2, the raw traces of the pupil positions and diameters within one subject are shown as an example. In the eye movement suppression task (left), the movements of both eyes were effectively suppressed. Small pupillo-constrictions were noted at about 400 ms after the onset of the near LED. The latencies were consistent with the light reflex elicited by the light flux from the near LED. In the random-interval task (middle), convergence eye movements with small downward movements were consistently evoked about 200 ms after the onset of the near LED. When comparing these findings with those of the eye movement suppression task, the onsets of pupillo-constrictions during the random-interval task were timed in a similar fashion but had larger amplitudes. Therefore, it is likely that not only the light reflex system but also the near response system was involved in eliciting these pupilloconstrictions.
Figure 2: Representative raw data showing anticipatory convergence. The raw traces of the positions and the diameters of both pupils of subject #5. The results from the eye movement suppression task, the random-interval task, and the regular-interval task are displayed from left to right. In each column, the changes in the horizontal position of the right pupil (Rt. Horiz.), the horizontal position of the left pupil (Lt. Horiz.), the convergence angle (Conv.), the vertical position of the left pupil (Lt. Vert.), the vertical position of the right pupil (Rt. Vert.), the diameter of the left pupil (Lt. Pupil), and the diameter of the right pupil (Rt. Pupil) are plotted from top to bottom. Upward deflection indicates rightward motion, convergence, upward motion, or pupillo-dilatation. Onset of the near LED is indicated by the vertical lines. The horizontal scales indicate 0.5 s, the vertical scales indicate 3 degree (eye movements) or 0.33 mm (pupil diameters). All data recorded within a single session were superimposed (n=49 in the eye movement suppression and the regular-interval tasks; n=13 in the random-interval task), without filtering. Our eye tracking system consistently and accurately detected the edge of the pupil almost throughout the whole experiment, but occasionally failed to do so because of blinks or obstructions by cilli (e.g. arrows).
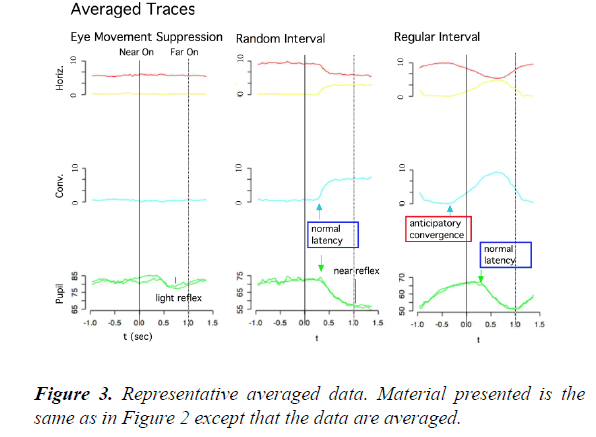
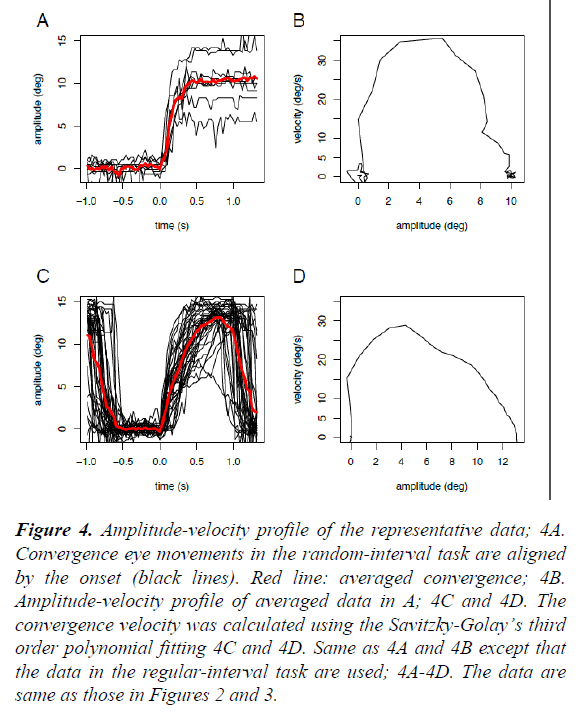
In contrast, in the regular-interval task (right), the onset times of convergence eye movements varied widely and frequently preceded the onset times of the near LED (anticipatory convergence), despite the subject not being instructed “ to precede” or “to anticipate” the onset of the near LED. On the other hand, the onset times and time courses of pupillary responses were similar to those in the random-interval task. We averaged the collected raw data from 49 or 13 trials in the same task due to the limited spatial and time resolutions of our video recording system (Figure 3). As shown in Figure 4, the amplitude-velocity profiles of the convergence eye movements elicited in the random-interval (Figure 4B) and regular-interval (Figure 4D) tasks were similar.
Figure 4: Amplitude-velocity profile of the representative data; 4A. Convergence eye movements in the random-interval task are aligned by the onset (black lines). Red line: averaged convergence; 4B. Amplitude-velocity profile of averaged data in A; 4C and 4D. The convergence velocity was calculated using the Savitzky-Golay’s third order polynomial fitting 4C and 4D. Same as 4A and 4B except that the data in the regular-interval task are used; 4A-4D. The data are same as those in Figures 2 and 3.
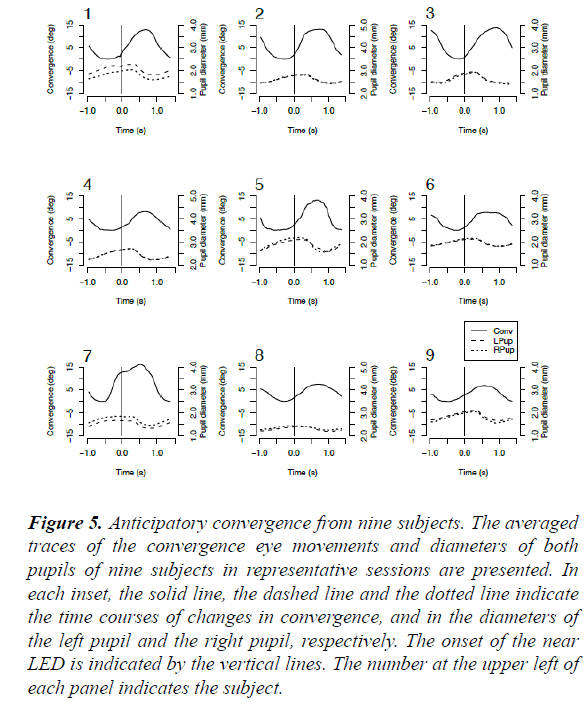
Discrepancies between the onset times of the convergence eye movement and pupillo-constriction, respectively, in the regular-interval task were consistently found among all nine subjects (Figure 5). Further, the mean onset times of the averaged convergence eye movements in the regular-interval task were significantly different from those in the randominterval task (one-way analysis of variance; F=19.1525; p<0.0001), whereas the difference between the mean onset times of the average pupillo-constriction in the regular- and random- interval tasks respectively, was not significant (F=1.4468; p=0.23) (Table 1).
Figure 5: Anticipatory convergence from nine subjects. The averaged traces of the convergence eye movements and diameters of both pupils of nine subjects in representative sessions are presented. In each inset, the solid line, the dashed line and the dotted line indicate the time courses of changes in convergence, and in the diameters of the left pupil and the right pupil, respectively. The onset of the near LED is indicated by the vertical lines. The number at the upper left of each panel indicates the subject.
Discussions
Slow eye movements, including both smooth pursuit and convergence eye movements, have been classically understood in the terms of being a part of a feedback control mechanism that minimizes the retinal error of the visual target within the parafoveal region [14]. In this study, however, we found that human subjects consistently performed anticipatory convergence eye movements when the target onset was predictable (Figures 2 and 3) with a similar amplitude-velocity profile (Figure 4). This result suggests that the anticipatory convergence shared the same motor command generator as that of the normal convergence eye movement, in agreement with the findings of previous investigations in humans [15,16] and cats [13].
The second finding was the existences of a discrepancy between the onset time of the convergence eye movement and the onset time of the pupillo-constriction in the anticipatory convergence, irrespective of the subject’s age. In the context of normal convergence, Myers and Stark reported that the latency of pupillary response was less affected by adding near response cues than were the latencies of either convergence or accommodation [17].
Our findings are in good agreement with their results. Besides, a discrepancy between blur-induced accommodation and pupillo-constriction was reported in humans by many researchers such as Stakenburg et al. [18], Phillips et al. [19], and Takagi et al. [20]. Combined with the outcomes of these previous studies, our findings suggest that the functional linkages between pupillo-constriction and the other two components of the near response are weaker than the functional linkage between the convergence eye movementrelated and lens accommodation-related systems.
In cats, it is known that among the three components of near response, only the output signals from the lateralsylvian area (Clare-Bishop area), one of the higher visual cortices in which the near response center exists [13,21,22], are transmitted indirectly via the area 20 to the midbrain pupillo-constriction-related areas [23]. Therefore we hypothesized that the ocular convergence-related areas and lens accommodation-related areas might receive more commands from higher cortical areas than the pupillo-constriction-related areas do. This hypothesis was supported by Gamlin and Mays who claimed that the convergence eye movement system is evolutionary newer than the pupillo-constriction system [24].
Conclusion
Since the difference between onset times of the convergence eye movement was less than 1 s, study modalities with a fine time resolution, such as electroencephalogram, electrocorticogram [25-28], or magneto-encephalogram [29] for example, may be required for further investigation of the neuronal bases of the discrepancy between the dynamics of the convergence system and the pupillo-constriction system in human, in addition to clinical studies.
References
- Jampel ST. Convergence, divergence, pupillary reactions and accommodation of the eyes from faradic stimulation of the macaque brain. J Comp Neurol. 1960;115:371-400.
- Feil M, Moser B, Abegg M. The interaction of pupil response with the vergence system. Graefes Arch Clin Exp Ophthalmol. 2017;255:2247-53.
- Semmlow J, Stark L. Pupil Movements to Light and Accommodative Stimulation : A Comparative Study. Vision Res. 1973;13:1087-100.
- Ukai K, Kato Y. The use of video refraction to measure the dynamic properties of the near triad in observers of a 3-D display. Ophthalmic Physiol Opt. 2002;22:385-8.
- Tondel GM, Candy TR. Accommodation and vergence latencies in human infants. Vision Res. 2008;48:564-76.
- Von Noorden K, Compos EC (2002) Binocular vision and ocular motility: Theory and management of strabismus. (6th edn), CV Mosby, St. Louis, London, UK.
- Nonaka F, Hasebe S, Ohtsuki H. Convergence Accommodation to Convergence (CA/C) Ratio in Patients with Intermittent Exotropia and Decompensated Exophoria. Jpn J Ophthalmol. 2004;48:300-5.
- Knoll HA. Pupillary changes associated with accommodation and convergence. Am J Optom Arch Am Acad Optom. 1949;26:346-57.
- Kasthurirangan S, Glasser A. Characteristics of pupil responses during far-to-near and near-to-far accommodation. Ophthalmic Physiol Opt. 2005;25:328-39.
- Kasthurirangan S, Glasser A. Age related changes in the characteristics of the near pupil responses. Vision Res. 2006;46:1393-403.
- Gislén A, Gustafsson J, Kröger RH. The accommodative pupil responses of children and young adults at low and intermediate levels of ambient illumination. Vision Res. 2008;48:989-93.
- Balaban CD, Kiderman A, Szczupak M, et al. Patterns of Pupillary Activity During Binocular Disparity Resolution. Front Neurol. 2018;26:990.
- Toda H, Tanimoto N, Takagi M, et al. Visual Cortical contribution to open-loop and feed-back control of convergence eye movements in the cat. Neurosci Res. 2006;54:302-12.
- Hung GK (2001) Models of oculomotor control. World scientific, Singapore.
- Kumar AN, Han Y, Garbutt S, et al. Properties of anticipatory vergence responses. Invest Ophthalmol Vis Sci. 2002;43:2626-32.
- Kumar AN, Han Y, Ramat S, et al. Anticipatory saccadic-vergence in humans. Ann N Y Acad Sci. 2002;956:495-8.
- Myers GA, Stark L. Topology of the near response triad. Ophthalmic Physiol Opt. 1990;10:175-181.
- Stakenburg M. Accommodation without pupillary constriction. Vision Res. 1991;31:267-273.
- Pillips NJ, Winn B, Gilmartin B. Absence of pupil response to blur-driven accommodation. Vision Res. 1992;32:1775-9.
- Takagi M, Abe H, Toda H, et al. Accommodative and pupillary responses to sinusoidal target depth movement. Ophthalmic Physiol Opt. 1993;13:253-7.
- Toyama K, Komatsu Y, Kozasa T. The responsiveness of Clare-Bishop neurons to motion cues from motion stereopsis. Neurosci Res. 1986;4:83-109.
- Toyama K, Fujii K, Kasai S, et al. The responsiveness of Clare-Bishop neurons to size cues from motion stereopsis. Neurosci Res 1986;4:110-128.
- Bando T, Norita M, Hirano T, et al. Projections of the postero-medial lateral suprasylvian area (PMLS) to cortical area 20 in cats with special reference to pupillary constriction: a physiological and anatomical study. Brain Res. 1989;494:369-73.
- Gamlin PD, Gnadt JW, Mays LE. Abducens Internuclear Neurons Carry an Inappropriate Signal for Ocular Convergence. J Neurophysiol. 1989;62:70-81.
- Toda H, Suzuki T, Sawahata H, et al. Simultaneous recording of ECoG and intracortical neuronal activity using a flexible multichannel electrode-mesh in visual cortex. Neuroimage. 2011;54:203-12.
- Toda H, Kawasaki K, Sato S, et al. Locally induced neuronal synchrony precisely propagates to specific cortical areas without rhythm distortion. Sci Rep. 2018;8:7678.
- Matsuo T, Kawasaki K, Osada T, et al. Intrasulcal electrocorticography in macaque monkeys with minimally invasive neurosurgical protocols. Front Syst Neurosci. 2011;5:34.
- Nakahara K, Adachi K, Kawasaki K, et al. Associative-memory representations emerge as shared spatial patterns of theta activity spanning the primate temporal cortex. Nat Commun. 2016;7:11827.
- Adams RA, Bauer M, Pinotsis D, et al. Dynamic causal modelling of eye movements during pursuit: Confirming precision-encoding in V1 using MEG. Neuroimage. 2016;132:175-189.