Original Article - Archives of Digestive Disorders (2017) Archives of Digestive Disorders (Special Issue 1-2017)
Differential effects of lipoteichoic acid and lipopolysaccharides on gastric cancer cell proliferation via distinctive involvement of TLR2 signaling mediators.
Emily KY Lam1,3, Emily KK Tai1, William KK Wu2,3,4, L Yu5, Helen PS Wong6, Vivian Y Shin7, CH Cho8*1Department of Pharmacology & Pharmacy, The University of Hong Kong, HKSAR, China
2Department of Anesthesia and Intensive Care, The Chinese University of Hong Kong, HKSAR, China
3State Key Laboratory of Digestive Diseases, Institute of Digestive Diseases, The Chinese University of Hong Kong, HKSAR, China
4Li Ka Shing Institute of Health Sciences, The Chinese University of Hong Kong, HKSAR, China
5State Key Laboratory of Organ Failure Research, Guangdong Provincial Key Laboratory of New Drug Screening, School of Pharmaceutical Sciences, Southern Medical University, Guangzhou, China
6Department of Chemistry, The University of Hong Kong, HKSAR, China
7Department of Surgery, The University of Hong Kong, HKSAR, China
8School of Biomedical Sciences, The Chinese University of Hong Kong, HKSAR
- *Corresponding Author:
- Prof. CH Cho
School of Biomedical Sciences
Faculty of Medicine
The Chinese University of Hong Kong
Shatin, Hong Kong
China
Tel: +852-3943 6886
Fax: +852-2603 5139
E-mail: chcho@cuhk.edu.hk
Accepted Date: March 22, 2017
Citation: Lam EKY, Tai EKK, Wu WKK, et al. Differential effects of lipoteichoic acid and lipopolysaccharides on gastric cancer cell proliferation via distinctive involvement of TLR2 signaling mediators. Arch Dig Disord. 2017;1(1):22-27.
Abstract
Toll like receptors (TLRs) are pattern-recognition receptors that recognize pathogen-associated molecular patterns. TLR signaling activates the innate immune response and induces the adaptive immunity, suggesting its significance to be a target for anti-cancer immunotherapy. In this study, we show that lipoteichoic acid (LTA) derived from gram positive Streptococcus faecalis markedly inhibited AGS gastric cancer cell proliferation. This is likely attributed to the induction of TNF-α through the upregulation of TLR2 expression and its downstream mediators including cytosolic adapter molecule myeloid differentiation protein MyD88, TNF receptor-associated factor 6 (TRAF6) and NF-κB activation. Lipopolysaccharides (LPS) derived from gram-negative Escherichia coli, on the other hand, did not affect AGS cell proliferation and TNF-α expression. Interestingly, LPS also induced TLR2 and MyD88 protein expression without affecting the downstream TRAF6 expression and NF-κB activation. It is suggested that these microbial components derived from gram-positive and gram-negative bacteria elicited differential effects on gastric cancer cell proliferation. The ability of these microbial components to induce TRAF6 expression could be one of the crucial elements underlying its differential functional effects upon TLR2 induction.
Keywords
Chemoradiation, Gastric cancer, TNF-α expression, Lipopolysaccharides, Escherichia coli, Cytotoxins
Introduction
Gastric cancer is the third most common malignancy worldwide [1]. Though various methods including surgery, chemotherapy, radiation, and chemoradiation therapy have been applied for the treatment of the disease, it remains the second leading cause of cancer-related death [2]. Alternative therapies are therefore aspired to treat gastric cancer. Immunotherapy is one of the novel therapeutic approaches for the treatment of malignant diseases and induction of the adaptive immunity has been suggested to be crucial for the treatment [3,4].
Toll like receptors (TLRs) are pattern-recognition receptors (PRR) that play crucial roles in host defense against invading microorganisms by recognizing pathogen-associated molecular patterns (PAMPs) [5]. They are comprised of an extracellular leucine-rich repeat (TRR) domain and an intracellular Toll/IL-1 receptor (TIR) domain [4,6]. The TIR domain is associated to several signaling components. Upon stimulation, the protein kinase IL-1 receptor-associated kinase (IRAK) is recruited to the receptor by the cytoplasmic myeloid differentiation protein MyD88 and phosphorylated [7]. The phosphorylated IRAK, in turn, interacts with tumor necrosis factor (TNF) receptorassociated factor 6 (TRAF6) [8] and results in the subsequent activation of downstream mediators including nuclear factor NF-κB, mitogen-activated protein kinases (MAPKs) and c-Jun/ AP1 [9].
Through the activation of the innate immune response and induction of the adaptive immunity [10], TLR signaling has been depicted as important mechanism and target for anticancer therapy. Microbial compounds are well known ligands of TLR that induce Th-1 cytokines via TLR2, TLR4 and TLR9 signaling [3,5,11]. Bacillus Calmette-Guérin cell wall skeleton (BCG-CWS) and peptidoglycan portion of BCGCWS have also reported to enhance cytotoxic activity of T cell and macrophages, and elicit an anti-tumor effect in rats [3], as well as induce cytokine production and enhance dendritic cells maturation through Toll signaling [12].
Lipoteichoic acid (LTA) is a surface-associated adhesion amphiphile derived from gram-positive bacteria which triggers the TLR signaling and subsequent cytotoxic activity [13]. In this study, we investigated the role of LTA for gastric cancer immunotherapy and examined the possible involvement of TLR signaling. Anti-cancer effects of Lipopolysaccharide (LPS), endotoxins from gram-negative bacteria, were also studied as it shares many pathogenic and cytotoxic similarities and with LTA.
Materials and Methods
Reagents and chemicals
All reagents and chemicals were purchased from Sigma (Sigma- Aldrich, USA) and all antibodies were purchased from Santa Cruz (Santa Cruz Biotechnology, USA) unless otherwise stated. Lipoteichoic acid (LTA) was derived from Streptococcus faecalis while Lipopolysaccharides (LPS) was derived from Escherichia coli. They were at least 97% pure from the lyophilized powder.
Cell culture and drug treatment
Human gastric adenocarcinoma cell line AGS (CRL-1739, ATCC, USA) was used. Cells were cultured in RPMI 1640 (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA), 100 U/ml penicillin G, 100 μg/ml streptomycin, and incubated in a incubator at 37°C, 95% humidity and 5% CO2. Cells were seeded in 24-well plates at a density of 2 × 104 cells per well in antibiotics-free culture medium. After 24 h, LTA or LPS at a dose of 0-50 μg/ml in FBS- and antibiotics-free medium was incubated with the cells for 24 h. All the experiments were performed with 3 repeats and analyzed.
[3H]-thymidine incorporation assay
A modified [3H]-thymidine incorporation assay was used to determine the amount of DNA synthesis as previously described [14]. Briefly, cells were incubated in the absence or presence of LTA or LPS for 24 hours and incubated with 0.5 μCi/ml [3H]- thymidine (Amersham Corporation, USA) for another 5 h. It was then washed with ice-cold 0.15 M NaCl and incubated with 10% trichloroacetic acid for 15 min at room temperature. The cells were incubated at 37°C for another 15 min after substantial washings. Next, cells were collected and hydrophilic scintillation fluid was added into the vial and the amount of DNA synthesized was measured using liquid scintillation spectrometry on a β-counter (Beckman Instruments, USA).
Semi-quantitative PCR
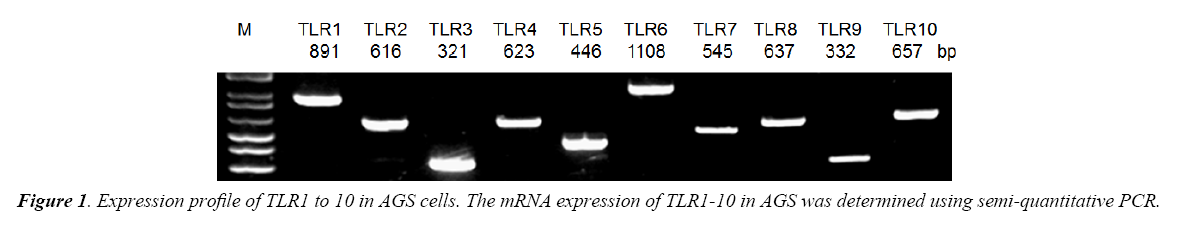
TLR1 to 10 mRNA expressions were determined by semiquantitative Revere Transcription-Polymerase Chain Reaction (RT-PCR). Total RNA was extracted from tissue using TRIzol reagent (Invitrogen, Carlsbad, CA). Five micrograms of total RNA were reverse-transcribed using the Thermoscript RT-PCR system reagent (Invitrogen, Carlsbad, CA). PCR amplification was carried out using the cDNA samples. Specific primers were designed for screening for the expression of TLR1 to 10. The PCR conditions for semi-quantification were as follows: the template cDNA was first denatured at 94°C for 5 min. During amplification, the denaturation step was at 94°C for 45 s, annealing at 55°C for 35 s for 35 cycles, and extension at 72°C for 10 min.
Extraction of total, cytosolic and nuclear protein
AGS cells were treated with either 20 μg/ml of LTA or LPS in FBS- and antibiotics-free medium for 0, 3, 6, 12 or 24 h. Total protein was extracted in radioimmunoprecipitation assay buffer containing proteinase and phosphatase inhibitors as previously described [14]. Protein was quantified by a protein assay kit (Bio-Rad Laboratories, USA) using BSA as standard.
Extraction of cytosolic and nuclear proteins was performed and modified as previously described [15]. Cells were harvested in hypotonic buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 0.5 mM PMSF, 1 mM NaF, 1 mM Na3VO4, 10 mM aprotinin, 1 μg/ml leupeptin and 1 μg/ml pepstatin A), lysed with 10% Nonidet P-40 and pelleted with supernatants collected as cytosolic extract. The remaining pellet was resuspended in extraction buffer (20 mM HEPES, pH 7.9, 1.5 mM MgCl2, 450 mM NaCl, 0.5 mM DTT, 25% glycerol, 1 mM EDTA, 0.5 mM PMSF, 1 mM NaF, 1 mM Na3VO4, 10 mM aprotinin, 1 μg/ml leupeptin and 1 μg/ml pepstatin A), agitated and centrifuged with supernatants collected as nuclear extracts.
Western blot analysis
Proteins (50 μg) were separated by electrophoresis on a 10% SDS-acrylamide gel, and transferred to Hybond C nitrocellulose membranes (Amersham International, UK). The membranes were then probed with anti-TLR2, anti-TLR4, anti-MyD88, anti-TRAF6, anti-NF-κB p65 or anti-TNF-α antibodies overnight at 4°C, incubated 1 h with secondary peroxidaseconjugated antibodies, and visualized with an enhanced chemiluminescence system (Amersham Biosciences, USA). Membranes were stripped with stripping solution (15 M Urea, 0.05 M Tris, 0.7% β-mercaptoethanol) for 30 min and re-probed with antibody against β-actin as an internal control.
Results
LTA inhibited AGS cell proliferation
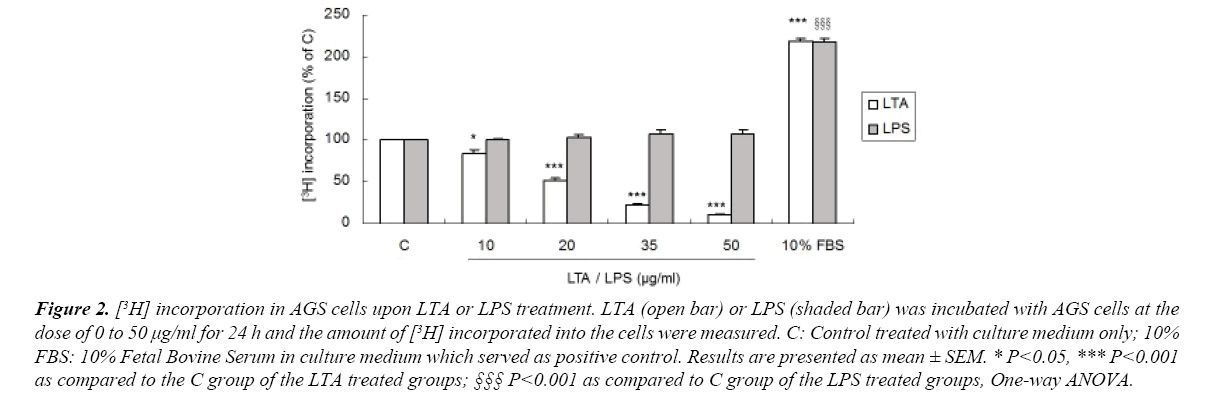
AGS cells were treated with LTA or LPS and their effects on cell proliferation were measured by the amount of [3H] incorporated into the cells. Treatment with LTA (10-50 μg/ml) markedly reduced cell proliferation in a dose-dependent manner (Figures 1 and 2), with inhibitory effects ranging from 18.6% to 89.9%. Ten percent FBS was used as positive control which stimulated cell proliferation. However, LPS at the same concentrations did not affect cell proliferation (Figure 2). These results showed that LTA and LPS derived from gram-positive and gramnegative bacteria, respectively, elicit a differential effect on gastric cancer cell proliferation. For the following experiments, LTA and LPS at the concentration of 20 μg/ml was used as LTA at this concentration reduced AGS cell proliferation to approximately 50%.
Figure 2. [3H] incorporation in AGS cells upon LTA or LPS treatment. LTA (open bar) or LPS (shaded bar) was incubated with AGS cells at the dose of 0 to 50 μg/ml for 24 h and the amount of [3H] incorporated into the cells were measured. C: Control treated with culture medium only; 10% FBS: 10% Fetal Bovine Serum in culture medium which served as positive control. Results are presented as mean ± SEM. * P<0.05, *** P<0.001 as compared to the C group of the LTA treated groups; §§§ P<0.001 as compared to C group of the LPS treated groups, One-way ANOVA.
LTA and LPS increased TLR2 but not TLR4 expression
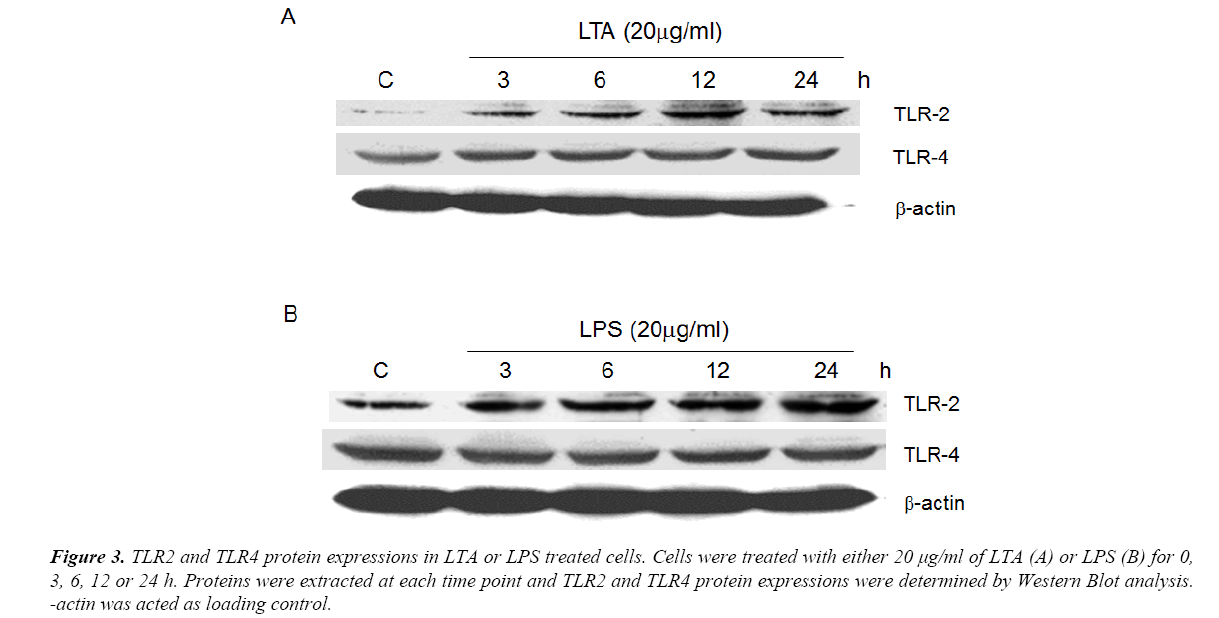
Using semi-quantitative RT-PCR, it was found that AGS cell line expressed all human TLR1 to 10 (Figure 1), indicating that it shared similar TLRs expression profile as human cells. As both LTA and LPS are reported ligands of TLR2 and TLR4 [16-18], Western Blot analysis were performed to delineate the role of TLR2 and TLR4 in LTA-induced anti-cell proliferative effect. LTA (20 μg/ml) and LPS (20 μg/ml) both time-dependently upregulated TLR2 protein expression. The maximal increase in TLR2 expression by LTA and LPS were observed after 12 h and 24 h of treatment, respectively (Figure 3). In contrast, the expression level of TLR4 was not altered by either treatment (Figure 3), suggesting the important or dominant involvement of TLR2 upon LTA and LPS treatment.
Figure 3. TLR2 and TLR4 protein expressions in LTA or LPS treated cells. Cells were treated with either 20 μg/ml of LTA (A) or LPS (B) for 0, 3, 6, 12 or 24 h. Proteins were extracted at each time point and TLR2 and TLR4 protein expressions were determined by Western Blot analysis. -actin was acted as loading control.
LTA upregulated TNF-α protein expression
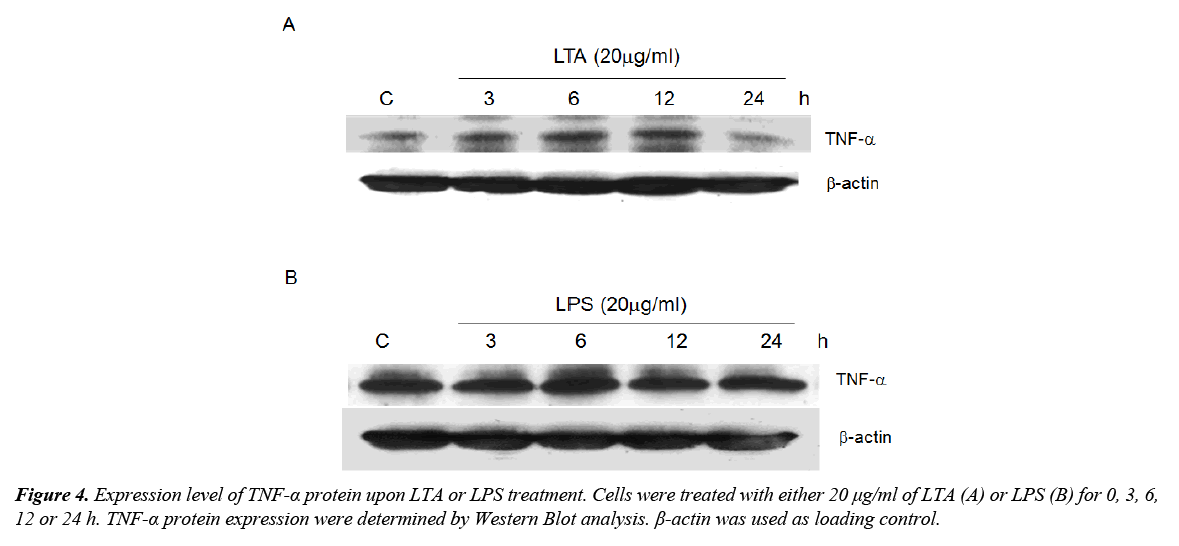
Activation of TLRs results in the synthesis of cytokines including TNF-α, which is a well-known anti-tumor factor. Since TLR2 was induced by both LTA and LPS, the role of these microbial components in regulating TNF-α expression was determined. Treatment with LTA time-dependently increased TNF-α expression with maximum expression level observed after 12 h of treatment (Figure 4A). LPS, on the other hand, did not alter TNF-α expression (Figure 4B). We postulated that LTA-induced anti-cell proliferation could be attributed to the induction of TNF-α whereas LPS failed to induce TNF-α and elicit similar functional effect.
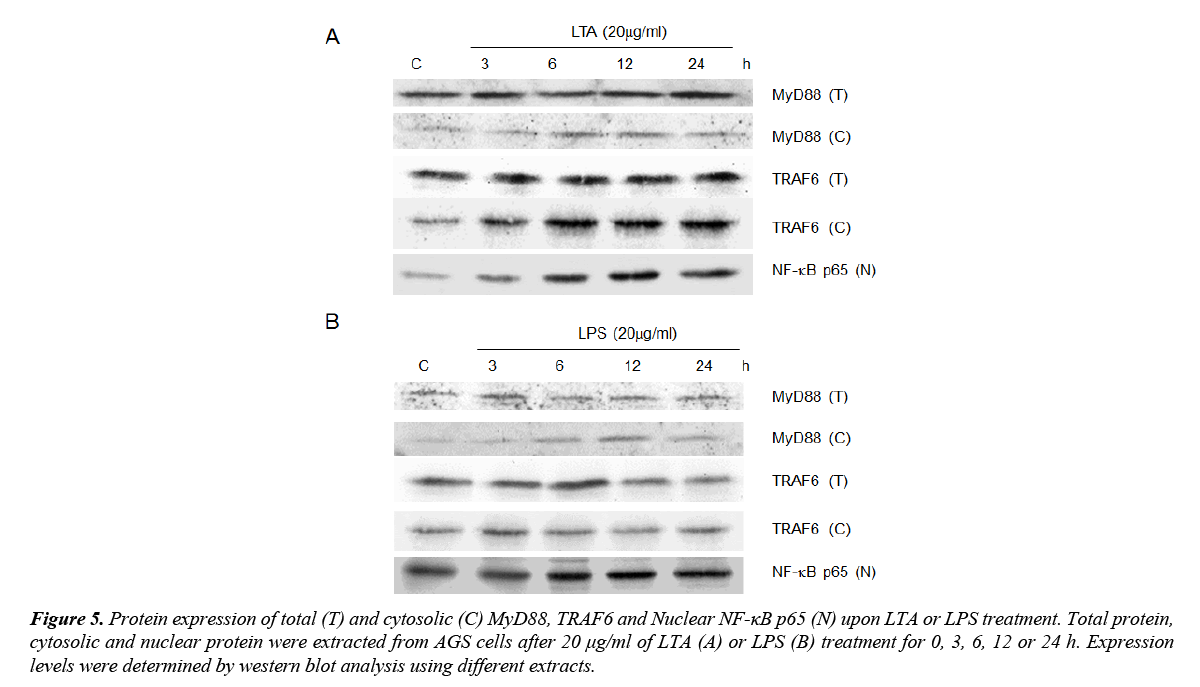
LTA and LPS on MyD88/TRAF6 and NF-κB signaling
MyD88, TRAF6 and NF-κB are downstream signaling mediators of TLR2 signaling. As both LTA and LPS induced TLR2 expression, the involvement of these signaling adaptors on LTA/LPS-induced TLR2 signaling was investigated. Using total protein extracted, neither MyD88 nor TRAF6 expression was altered by LTA/LPS treatment (Figure 5). However, both LTA and LPS up-regulated cytosolic MyD88 expression (Figure 5). The maximal effect exerted by LTA was observed at 6 and 12 h (Figure 5A) whereas that for LPS was at 12 h after treatment (Figure 5B). However, cytosolic TRAF6 was only up-regulated by LTA with sustained high expression levels after 6 h or longer treatment time (Figure 5). Furthermore, nuclear NF-κB p65 was time-dependently up-regulated by LTA (maximal expression at 12 and 24 h) but not LPS (Figure 5). Since activation of NF-κB is downstream of TRAF6 and is critical for TNF-α induction, the inability of LPS on stimulating TRAF6 expression could be one of the crucial elements underlying its differential effects on TNF-α expression and cell proliferation upon TLR2 induction as compared to that of LTA.
Figure 5. Protein expression of total (T) and cytosolic (C) MyD88, TRAF6 and Nuclear NF-κB p65 (N) upon LTA or LPS treatment. Total protein, cytosolic and nuclear protein were extracted from AGS cells after 20 μg/ml of LTA (A) or LPS (B) treatment for 0, 3, 6, 12 or 24 h. Expression levels were determined by western blot analysis using different extracts.
Discussion and Conclusion
This study is to compare the differential effects between LTA and LPS from different strains of bacteria on cancer cells. The expressions of different immuno-modulators in cells are compared between the two cytotoxins in a relative manner in order to elucidate their differential actions on cancer development. We found that LTA significantly reduced gastric cancer cell proliferation, which is likely associated with the induction of TNF-α expression that initiate inhibitory effects on cell proliferation. Similar effects were not produced by LPS, although it shares many pathogenic properties with LTA. This suggested that these microbial components originated from gram-positive and gram-negative bacteria react differently at least in AGS cells. Since LTA triggers immune responses in many ways such as inducing the release of reactive oxygen and nitrogen species, acid hydrolases, highly cationic proteinases, bactericidal cationic peptides, growth factors, and cytotoxic cytokines [19], it is possible that LTA reduced gastric cancer cell proliferation through synergistic effects of all these elements. Nevertheless, we postulated TNF-α as one of the crucial elements as reflected by its high expression levels upon LTA treatment.
The association between TLRs and its respective ligands has been demonstrated to result in anti-cancer effects through the induction of Th-1 T-cell responses, enhancement of the cytotoxic activity of macrophages, and maturation of dendritic cells [3,11,12]. We found that both LTA and LPS up-regulated TLR2 but not TLR4 protein expression, suggesting that TLR2 plays a dominant role in the recognition of these bacterial components at least in our system. Indeed, TLR2 was known to recognize LTA [20,21], LPS [18], peptidoglycan [17], lipoprotein [22] and lipoarabinomannan [23] whereas TLR4 recognize LPS [16], LTA [24], fibronectin fragment [25] and heat shock protein 60 [26]. However, the expression level of TLR4 was not altered by either LPS or LTA treatment. It remains unknown why LPS failed to induceTLR4, a well known ligand-receptor recognition system. It is speculated that different cell lines and culture systems could have different ligand-receptor responses.
Some studies have demonstrated the involvement of TLR4 in the anti-tumor effect induced by dendritic cells immunotherapy and by LTA-related molecules [27,28]. However, LTA-related molecule was found to elicit anti-cancer activity even in TLR4- deficient host [29]. These findings implicated that the presence and activation of TLR4 may not be essential for the anti-cancer effect exerted by LTA [30]. It is likely that the LTA-induced anti-cancer cell proliferation effect is independent of TLR4 but TLR2 in the current system.
MyD88, TRAF6 and NF-κB are downstream signaling mediators of TLR2 signaling. Upon stimulation, MyD88 recruits the protein kinase IL-1 receptor-associated kinase (IRAK), which interacts with TNF receptor-associated factor 6 (TRAF6) after phosphorylation and results in the activation of the transcriptional factor NF-κB [7-9], which subsequently leads to the induction of Th-1 type T-cell response, activation of killer cells, and production of various cytokines including TNF-α. Our results showed that cytosolic MyD88 expression was upregulated by both LTA and LPS in a time-dependent manner, indicating that MyD88 could be involved in the signaling cascade although it was not an essential factor for TLR signaling [29,30].
It was also found that LTA but not LPS up-regulated cytosolic TRAF6 and the downstream nuclear NF-κB p65. Theoretically, upregulation of TLR2 should result in substantial increase of TNF-α production. Despite of the ability of both microbial components to up-regulate TLR2 expression, only LTA was found to induce TNF-α production and inhibit AGS cell proliferation. It is postulated that LTA induce TLR2 and its signaling mediators including MyD88, TRAF6 and NF-κB p65, which result in the production of TNF-α and lead to the inhibitory effect on gastric cancer cell proliferation. Similar study using LTA from Stretococcus pneumonia and Staphylococcus aureus confirms that TLR2 but not TLR4 was involved in the activation of immune cells [13]. LPS, on the other hand, also induce TLR2 and the downstream MyD88 without altering other signaling mediators such as TRAF6 and NF-κB p65 and interrupt the downstream production of TNF-α and the subsequent antiproliferative effects. It could be the interruption or disturbance of TRAF6 or/and NF-κB p65, through unknown mechanisms, that resulted in distinctive effects produced by LTA and LPS. Studies in MyD88- and TRAF6-deficient mice had revealed that TRAF6 but not MyD88 was necessary for NF-κB activation [30-32], it is likely that TRAF6 or/and NF-κB represent(s) as vital mediator(s) in TLR2 signaling by LTA. This study has shed new light on future development of anticancer drugs using bacterial compounds, which are capable to induce antiproliferative effects by activating the hosts’ own immune responses, and could diminish severe side effects limited by the current chemotherapies in clinical practice.
Acknowledgment
This work was supported by the Health and Medical Research Fund (13120062) from the Food & Health Bureau and the General Research Fund (CUHK463613) from the Research Grant Council of Hong Kong and also the National Natural Science Foundation (81473269) from China.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN. Int J Cancer. 2015;136(5):E359-86.
- Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1-9.
- Okamoto M, Sato M. Toll-like receptor signaling in anti-cancer immunity. J Med Invest. 2003;50:9-24.
- Shen J, Li ZJ, Li LF, et al. Vascular-targeted TNFα and IFNγ inhibits orthotopic colorectal tumor growth. J Transl Med. 2016;14(1):187.
- Leifer CA, Medvedev AE. Molecular mechanisms of regulation of Toll-like receptor signaling. J Leukoc Biol. 2016;100:927-41.
- Rock FL, Hardiman G, Timans JC, et al. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci U S A. 1998;95:588-93.
- Medzhitov R, Preston-Hurlburt P, Kopp E, et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253-8.
- Cao Z, Henzel WJ, Gao X. IRAK: A kinase associated with the interleukin-1 receptor. Science. 1996;271:1128-31.
- Muzio M, Mantovani A. Toll-like receptors. Microbes Infect. 2000;2:251-5.
- Medzhitov R, Preston-Hurlburt P. A human homologue of the drosophila toll protein signals activation of adaptive immunity. Nature. 1997;388:394-7.
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675-80.
- Azuma I, Seya T. Development of immunoadjuvants for immunotherapy of cancer. Int Immunopharmacol. 2001;1:1249-59.
- Schröder NW, Morath S, Alexander C, et al. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem. 2003;278(18):15587-94.
- Shin VY, Wu WK, Chu KM, et al. Functional role of β-adrenergic receptors in mitogenic action of nicotine on gastric cancer cells. Toxicol Sci. 2007;96:21-9.
- Chen CC, Wang JK, Lin SB. Antisense oligonucleotides targeting protein kinase C-alpha, -beta I, or -delta but not -eta inhibit lipopolysaccharide-induced nitric oxide synthase expression in RAW 264.7 macrophages: involvement of a nuclear factor kappa B-dependent mechanism. J Immunol. 1998;161:6206-14.
- Hoshino K, Takeuchi O, Kawai T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749-52.
- Schwandner R, Dziarski R, Wesche H, et al. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406-9.
- Yang RB, Mark MR, Gray A, et al. Toll like receptor-2 mediates lipopolysaccharide-induced cellular signaling. Nature. 1998;395:284-8.
- Ginsburg I. Role of lipoteichoic acid in infection and inflammation. Lancet Infect Dis. 2002;2:171-9.
- Michelsen KS, Aicher A, Mohaupt M, et al. The role of toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCS). Peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. J Biol Chem. 2001;276:25680-6.
- Morath S, Geyer A, Hartung T. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J Exp Med. 2001;193:393-7.
- Yoshimura A, Lien E, Ingalls RR, et al. Cutting edge: Recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1-5.
- Means TK, Lien E, Yoshimura A, et al. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J Immunol. 1999;163:6748-55.
- Dessing MC, Schouten M, Draing C, et al. Role played by Toll-like receptors 2 and 4 in lipoteichoic acid-induced lung inflammation and coagulation. J Infect Dis. 2008;197:245-52.
- Okamura Y, Watari M, Jerud ES, et al. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229-33.
- Ohashi K, Burkart V, Flohé S, et al. Cutting edge: Heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558-61.
- Ahmed SU, Okamoto M, Oshikawa T, et al. Anti-tumor effect of an intratumoral administration of dendritic cells in combination with TS-1, an oral fluoropyrimidine anti-cancer drug, and OK-432, a streptococcal immunopotentiator: involvement of toll-like receptor 4. J Immunother. 2004;27:432-41.
- Okamoto M, Oshikawa T, Ohe G, et al. Severe impairment of anti-cancer effect of lipoteichoic acid-related molecule isolated from a penicillin-killed Streptococcus pyogenes in toll-like receptor 4-deficient mice. Int Immunopharmacol. 2001;1:1789-95.
- Oshikawa T, Okamoto M, Ahmed SU, et al. Anti-cancer effect of an intratumoral injection of dendritic cells expressing TLR4 in combination with an active component of OK-432 in TLR4-deficient mice. Gan To Kagaku Ryoho. 2004;31:1770-2.
- Traore K, Zirkin B, Thimmulappa RK, et al. Upregulation of TLR1, TLR2, TLR4, and IRAK-2 expression during ML-1 cell differentiation to macrophages: Role in the potentiation of cellular responses to LPS and LTA. ISRN Oncol. 2012;2012:1-10.
- Kawai T, Adachi O, Ogawa T, et al. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:15-22.
- Lomaga MA, Yeh WC, Sarosi I, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015-24.