Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2024) Volume 14, Issue 106
Dietary Fiber Determination of Nanocrystals
Chaya G1*, Razia Sulthana2, Savithri K21Department of Studies in Chemistry, Bharathi College - Post Graduate and Research Centre, Bharathi Nagara-571422, Karnataka, India
2Department of Studies in Chemistry, University of Mysore, Manasa Gangothri, Mysore-570006, Karnataka, India
- *Corresponding Author:
- Chaya G. M
Department of Studies in Chemistry
Bharathi College - Post Graduate and Research Centre
Bharathi Nagara - 571422, Karnataka, India
E-mail: chayaguruswamy97@gmail.com
Received: 01-July-2024, Manuscript No. AABPS-24-145403; Editor assigned: 02-July-2024, Pre QC No. AABPS-24-145403(PQ); Reviewed: 15-July-2024, QC No. AABPS-24-145403; Revised: 19-July-2024, Manuscript No. AABPS-24-145403(R); Published: 26-July-2024, DOI: 10.35841/aabps-14.106.242
Citation: Chaya G, Razia Sulthana, Savithri K. Dietary fiber determination of nanocrystals. Asian J Biomed Pharm Sci.2024; 14(106):242
Keywords
Cellulose Nano crystal, Dietary fiber, Enzymatic hydrolysis, Biomaterials.
Introduction
In addition to being more cost-effective than comparable highperformance nanomaterials, nanocrystals can replace several goods derived from petrochemicals. Different qualities of CNCs are mostly caused by variations in the extraction process of CNCs. A primary drawback of using CNCs for commercial purposes is that they can be effectively fabricated at a costeffective quantity and quality [1]. The materials world has given cellulose nanocrystals (CNs) a great deal of attention, and this interest does not seem to be waning.
These biopolymeric assemblies demand such attention not only because of their outstanding core physical and chemical qualities (as will become obvious in the review) but also because of their intrinsic renewability and sustainability in addition to their abundance. They have been the focus of numerous research projects because of their low cost, availability, renewability, lightweight, nanoscale dimension, and distinctive shape as reinforcing agents in nanocomposites. CNs are the basic polymeric motifs that make up macroscopic fibers derived from cellulosic materials, whose sheer volume surpasses any known synthetic or natural biomaterial. Biopolymers, including cellulose, lignin, and occasionally heteropolysaccharides, offer the hierarchical frameworks needed to bioengineer biological factories that produce diverse plants and creatures. Surprisingly, it has only been in the last few years, with the availability of a virtual information repository, that attention has been directed to nanoscale phenomena involving these materials [2].
The method for categorizing the fibers in consumer food products is to do so according to the uses of the food and for labeling and regulatory purposes. DFs fall into two categories: "endogenous" and "added" fibers. The former describes the intrinsic, intact fibers found in the original food matrix, while the latter describes a collection of fibers extracted from specific sources and reincorporated into a product formulation. Dietary fiber is made up of lignin and indigestible carbohydrates that are inherent to plants and remain intact. Isolated, in digestible carbohydrates that have positive physiological effects on humans make up added fiber [3]. Fruits and vegetables, as well as wholegrain cereals, contain dietary fiber. The indigestible plant components that mostly pass through our stomachs and intestines undigested makeup fiber. Essentially, fiber is a carbohydrate. The majority of dietary fiber comes from the cell walls of plants. It is mostly composed of resistant starch, lignin, pectic polysaccharides, cellulose, and hemicellulose.
Cellulose nanocrystals (CNCs) are added to a variety of polymer matrices to produce reinforced composites. They are thought to be among the best nano-reinforcements for polymer matrices, including systems of polymers that are both watersoluble and water-insoluble [4]. Furthermore, the benefits of ecologically friendly CNCs include a low density, low energy consumption, biodegradability, biocompatibility, intrinsic renewability, and high aspect ratio [5].
It is possible to produce CNCs with extremely crystalline rod-shaped nanoparticles by enzymatic or controlled acid hydrolysis of various cellulose sources. When lignocellulosic biomass is exposed to a mix of chemical, mechanical, and/or enzymatic treatment, along with pure mechanical shearing, the amorphous portions of cellulose microfibrils are hydrolysed selectively under particular conditions [6-9]. This is because amorphous domains are more vulnerable to attack than crystalline ones. As a result, these microfibrils fragment into shorter, highly crystalline segments, commonly known as cellulose nanocrystals (CNCs).
Materials and Methods
The chemical reagents used were: sodium hypochlorite (SDFCL, S d fine-chem. Ltd., Mumbai), hydrochloric acid (HCl, RANKEM, AR grade, RFCL Limited, New Delhi), sodium hydroxide pellets (NaOH, Qualigens Fine Chemicals, Mumbai), α- amylase, pancreatin, pepsin (Sigma Aldrich, St. Louis, MO 63178, USA, 314-771-5750), sodium hydroxide pellets (AR grade, SDFCL, S d fine-chem. Ltd., Mumbai), disodium hydrogen orthophosphate anhydrous (AR, Qualigens, Mumbai), and sodium dihydrogen ortho-phosphate dehydrate (AR, SDFCL, S d fine-chem. Ltd., Mumbai). Without undergoing additional purification, all of the compounds utilised for analysis were of analytical quality.
Instruments/Equipments used for analysis
Dietary Fibre Filteration System Apparatus (CSF6, VELP SCIENTIFICA, Italy.), Temperature Controller Protronics Water Bath (SCIENCEMARK), Heated circulating water bath GDE, VELP SCIENTIFICA with multi-stirrer equipment, Muffle furnace, pH meter (Mettler Toledo), magnetic stirrer with hot plate TARSONS SPINOT DIGITAL, BENCH type pH meter with microprocessor with 3 points (EUTECH, pH tutors), nano-DLS instrument (MALVERN, U.K.), The structural analysis to determine functional groups was carried out by the instrument ATR-FTIR (Perkin-Elmer Spectrum 2).
Synthesis of cellulose nanocrystals
Chemical extraction of Nutraceutical Industrial Tribulus Terrestris Spent cellulose nanocrystals
100 g of NITTS was grounded in fibres (<177μ) and treated with 3% w/v of NaOH at 900 °C for 4 h with endless moving at 1200 rpm. Watmann 1 filtered paper was used to filter the solution following the alkali treatment. The resulting insoluble residue material was then processed further, utilising 1.7% w/v for a bleaching step. Following two hours of incubation at room temperature and a distilled water filter, NaOCl was added, dried, and filtered. Once more, 18% weight of NaOH was added, mixed for ten minutes, and then filtered. Ultimately, a fine, amorphous powder of NITTS cellulose nanocrystals was obtained by adding 5% w/v of HCl.
Dietary fiber determination of Nutraceutical Industrial Tribulus Terrestris Spent cellulose nanocrystals
1 g of grounded NITTS cellulose nanocrystals amorphous powder was added to a 0.1 M sodium phosphate buffer solution maintained at pH 6, and 100 mL of α-amylase was added. The incubation was done at 1000 °C in a temperaturecontrolled protronics water bath with constant stirring. Later cooled, add 20 ml of distilled water to the solution adjusted to pH-1.5 using 2N HCl. Add 100 mg of pepsin and incubate at 400 °C in a heated circulating water bath (GDE, VELP SCIENTIFICA with multi-stirrer equipment) for 1 h. Again cooled, add 20 ml of distilled water to the solution and adjust the pH to 6.3 using a 2N NaOH solution. Later, 100 mg of pancreatin was added to the solution and incubated at 400 °C in a water bath for 1 hour. Later, the solution was set to pH 4.5, and the solution was filtered using a dietary fibre filteration system with pressure and vacuum operated. While filtering, a hot solution of ethyl alcohol and acetone was used. Later, the obtained sample powder was dried in a hot air oven at 1010 °C. During the process, before and after drying, the sample weight was taken later incinerated in a muffle furnace. From dietary determination, the cellulose nanocrystal obtained a fibre content of 70%. The sample analysis was carried out in duplicate. The dietary fibre determination of NITTS adsorbent details is shown in (Table 1).
| Empty crucible weight | Celite weight | Before drying | After drying | Sample wt. taken | Ash content | % Fiber |
|---|---|---|---|---|---|---|
| 30.954 | 0.5034 | 30.234 | 30.563 | 0.6253 | 29.417 | 73.54 |
| 32.0006 | 0.5678 | 30.568 | 30.147 | 1.0099 | 30.467 | 70.50 |
Table 1. Dietary fiber determination of NITTS adsorbent.
Results and Discussion
Dynamic laser light scattering (DLS)
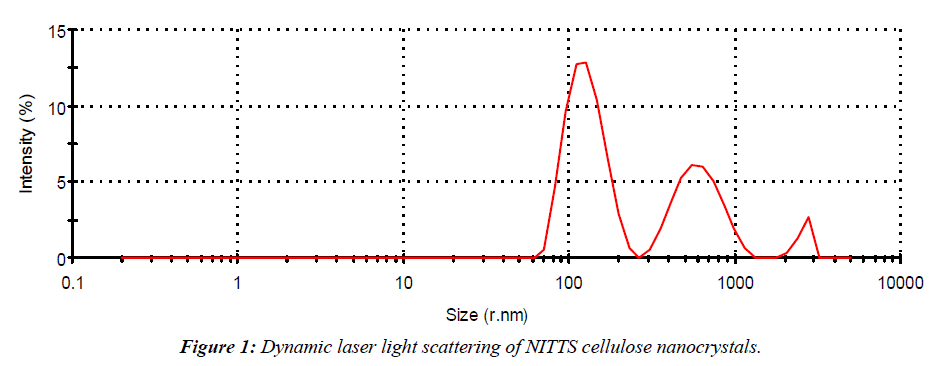
The size of NITTS cellulose nanocrystals was measured by Zetasizer Nano DLS (MALVERN, UK). 10 mg of cellulose nanocrystals were suspended in distilled water and kept in an ultrasonicator bath for 20 min. Afterwards, DLS analysis was used to ascertain the particle size. Size measurements were performed at 250 °C. The cellulose nanocrystals had an average diameter and intensity size determination by using dynamic light scattering. The size Z-average radius shows 210.7 and the polydispersivity index (PDI) shows 0.442 (Figure 1).
Scanning Electron Microscopy (SEM)
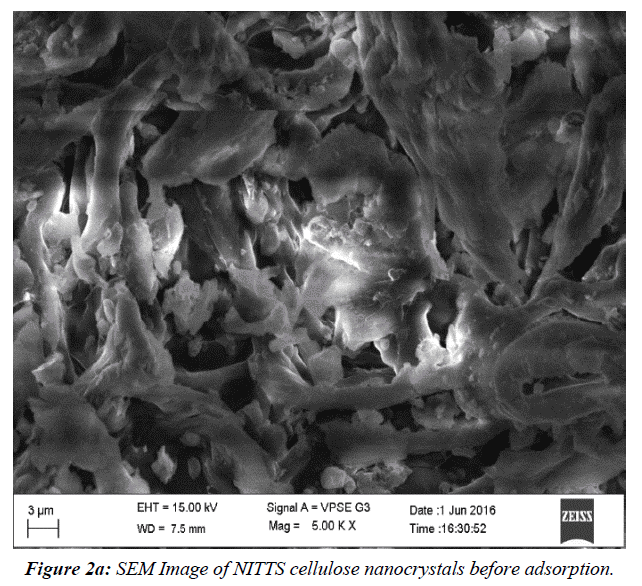
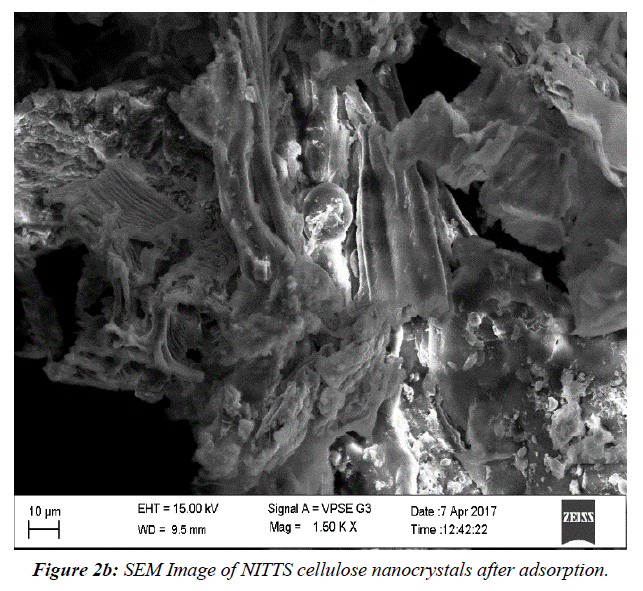
The NITTS's pliable and elastic configuration was revealed via SEM examination. This assembly facilitates better dye absorption. (Figure 2a) shows SEM Image of NITTS cellulose nanocrystals before adsorption. (Figure 2b) shows SEM Image of NITTS cellulose nanocrystals before adsorption. The porous structure indicates that there is adsorption of NITTS with cellulose nanocrystal.
Fourier Transform Infrared Spectroscopy (FTIR)
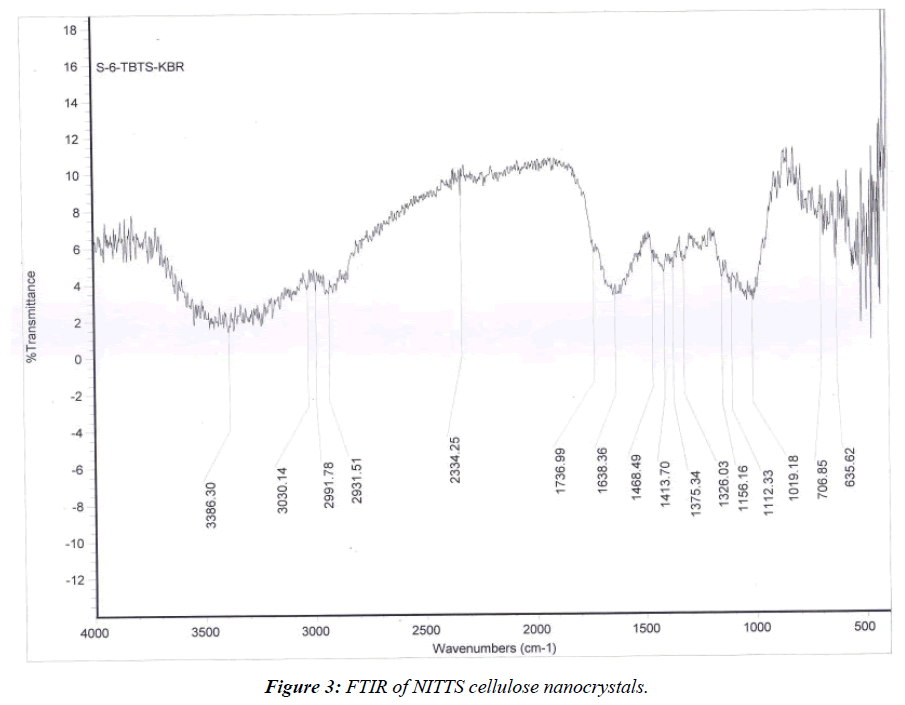
The FTIR spectra of preserved NITTS cellulose nanocrystal and untouched NITTS cellulose nanocrystal are in the range of 600-4000 cm-1. It is compared with other functional groups present in NITTS cellulose nanocrystal (Figure 3). The broad band in Figure 3 was assigned to the surface hydroxyl assemblies, connected in cellulose and adsorbed water, while the band at 2931 cm-1 was related to the enlarging tremors of -CH bonds in alkane and alkyl groups. While the band at 1737 cm-1 results from olefins being stretched C=C. A band at 1414 cm-1 indicates the occurrence of methyl groups. The existence of cellulose in the adsorbent was 1019 cm-1.
The elongating sensations of -CH bonds in alkane and alkyl groups, where carbon is connected with hydrogen bonds, were assigned to the bands after the adsorption shift of bands from 2931 to 2991 cm-1 indicated NITTS adsorption. This clearly shows that BG has been involved in adsorption. The stretch vibrations of the C=C bond in the quinonoid structure [10] of NITTS decreased in frequency from 1638 cm-1 to 1468 cm-1. There was no discernible shift in the frequency of the groups at 1414 cm-1 and 1375 cm-1, which are caused by the dye molecules C-N stretching and C-H deformation, respectively. The results showed that several peaks had moved or vanished, and new peaks had appeared. These variations in the spectrum suggested that those functional groups may have been involved in the adsorption process. Following the chemical alteration, the absorption groups among 1019 cm-1 and 707 cm-1 were different. To pinpoint the adsorbent and adsorbate groups in charge of the dye adsorption, FTIR spectroscopy is useful [11].
Conclusion
The preparation of cellulose nanocrystals was a simple method. Isolation of alkali, bleaching treatment, and acid hydrolysis were easy and simple methods to obtain fine residual amorphous cellulose nanocrystals. Enzymatic hydrolysis of NITTS cellulose nanocrystals is carried out through the dietary fibre method to determine the fibre percentage. After enzymatic hydrolysis, all proteins and starch were digested completely, with the exception of fibre powder, which might be cellulose microfibril content.
References
- Trache D, Hussin MH, Haafiz MM, Thakur VK. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale. 2017;9(5):1763-86.
- Habibi Y, Lucia LA, Rojas OJ. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem Rev. 2010;110(6):3479-500.
- Li YO, Komarek AR. Dietary fibre basics: Health, nutrition, analysis, and applications. Food Quali Safe. 2017;1(1):47-59.
- Cao Y, Wang HJ, Cao C, et al. Synthesis and anti-ultraviolet properties of monodisperse BSA-conjugated zinc oxide nanoparticles. Mater Let. 2011;65(2):340-2.
- Siró I, Plackett D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose. 2010 Jun;17:459-94.
- Beck-Candanedo S, Roman M, Gray DG. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomc Molec. 2005;6(2):1048-54.
- Bondeson D, Mathew A, Oksman K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose. 2006;13:171-80.
- Filson PB, Dawson-Andoh BE. Sono-chemical preparation of cellulose nanocrystals from lignocellulose derived materials. Biores Techno. 2009;100(7):2259-64.
- Filson PB, Dawson-Andoh BE, Schwegler-Berry D. Enzymatic-mediated production of cellulose nanocrystals from recycled pulp. Green Chem. 2009;11(11):1808-14.
- Har MR, Sathasivam K. The removal of methyl red from aqueous solutions using banana pseudostem fibers. Ameri J Appl Sci. 2009;6(9):1690-700.
- Collier R, Fuchs B, Walter N, et al. Ex vitro composite plants: an inexpensive, rapid method for root biology. The Plant Journal. 2005 Aug;43(3):449-57.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref