- Biomedical Research (2015) Volume 26, Issue 4
Diagnostic value of bronchial wash, bronchial brushing, fine needle aspiration cytology versus combined bronchial wash and bronchial brushing in the diagnosis of primary lung carcinomas at a tertiary care hospital
Shagufta Tahir Mufti*, Ghadeer A. MokhtarDepartment of Anatomic Pathology, Faculty Of Medicine, King Abdulaziz University, PO Box 80215,Jeddah 21589, Saudi Arabia
- *Corresponding Author:
- Shagufta Tahir Mufti
Department of Anatomic Pathology
Faculty of Medicine
King Abdulaziz University
PO Box 80215, Jeddah 21589
Saudi Arabia
E-mail: shagufta.mufti@gmail.com
Accepted date: August 12 2015
Abstract
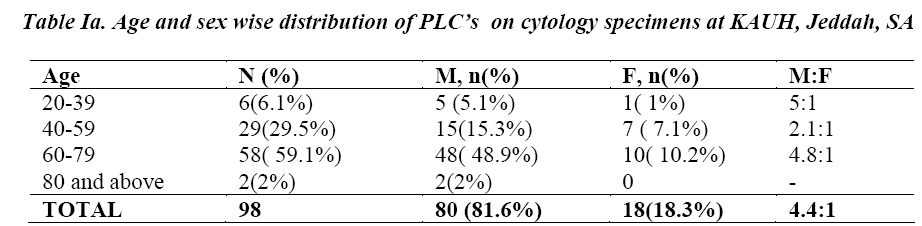
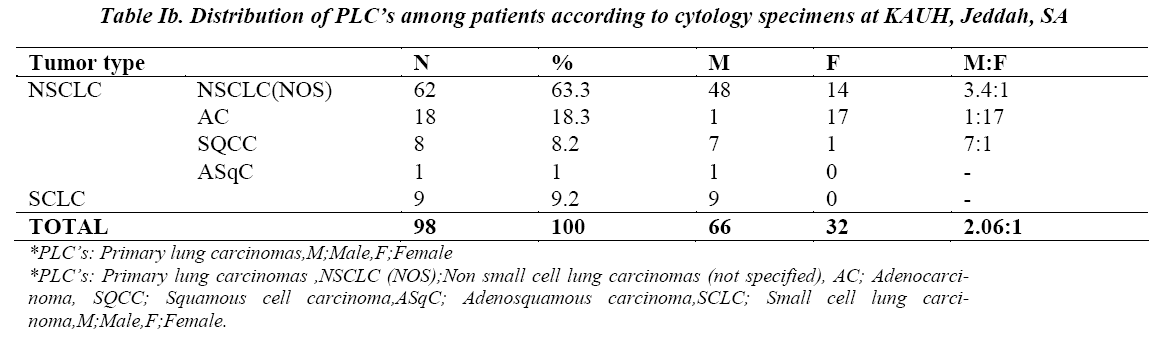
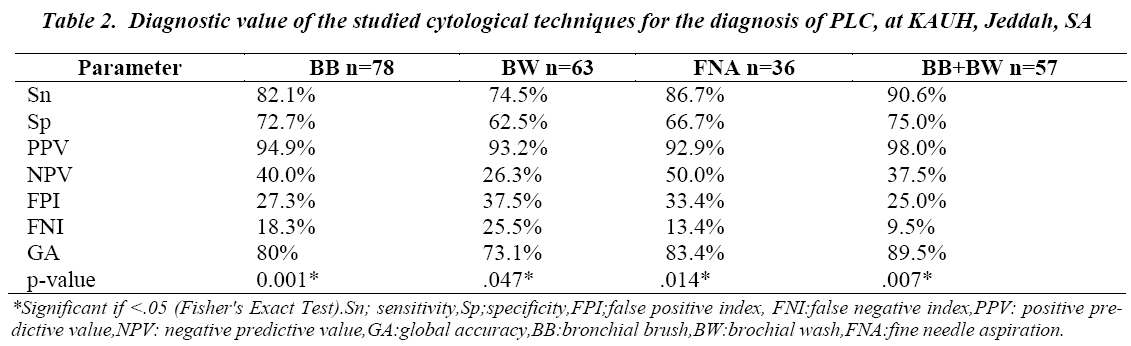
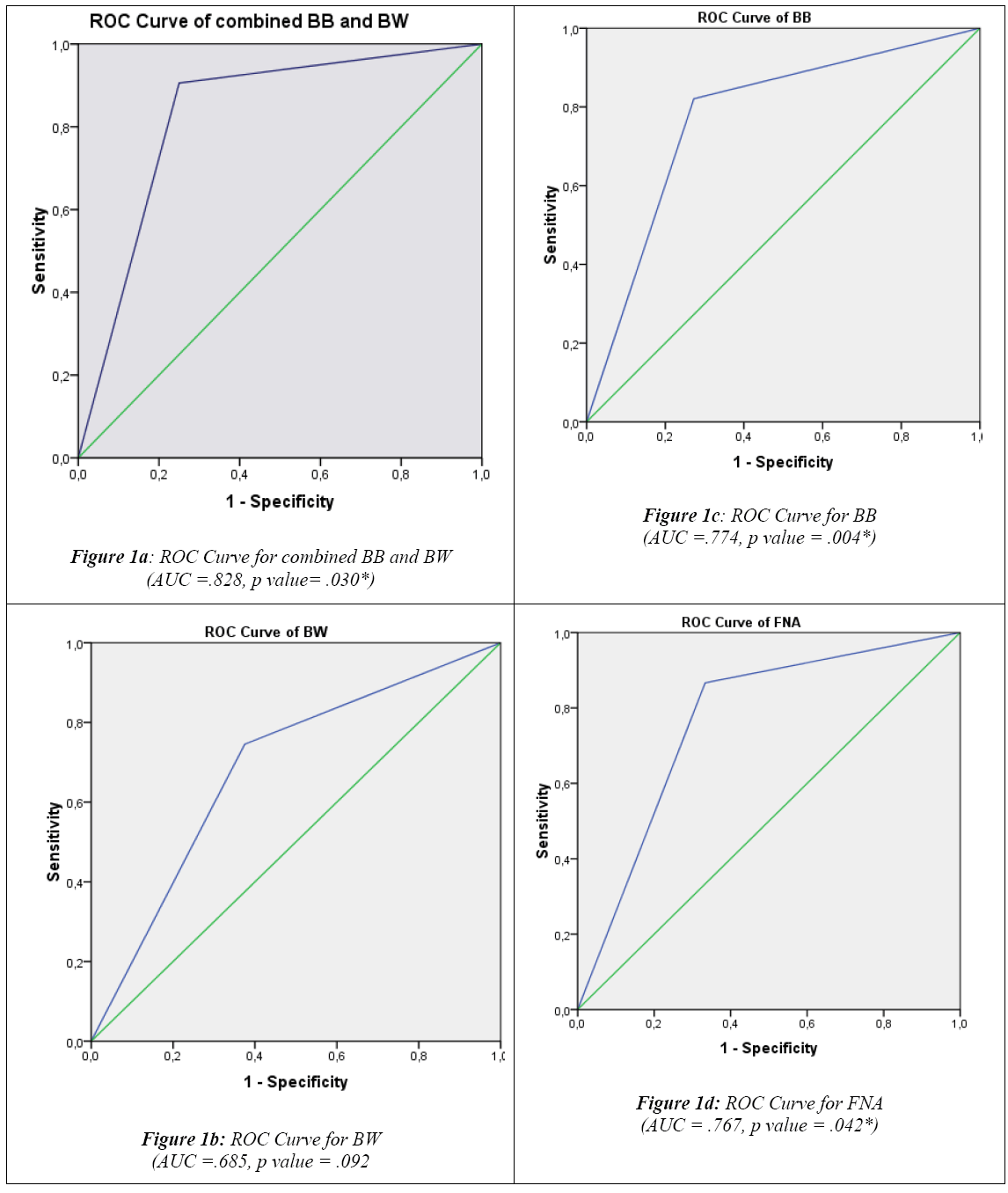
Different cytological techniques have been successfully applied for the diagnosis of primary lung cancers. Flexible fiberoptic bronchoscopy has served as a major breakthrough in respiratory cytology, as bronchial brushings, washings and fine needle aspiration have become more easy, accessible and cost effective.This study aims to determine the diagnostic value of bronchial wash (BW), bronchial brushing (BB) and fine needle aspiration (FNA) cytological samples in diagnosing primary lung carcinoma, among patients attending King Abdulaziz University Hospital, Jeddah Saudi Arabia. A retrospective analysis was performed to compare the diagnostic value of the three specimens’ types among patients with a clinical suspicion of primary lung carcinoma. All cytology specimens of bronchial washing, bronchial brushing and fine needle aspirate of lung performed for a clinical diagnosis of primary lung carcinoma, between Jan 2000- Dec 2013, were identified and evaluated in comparison to their respective histological correlations. Combined BB + BW showed the best sensitivity (90.6%), specificity (75%), PPV (98%) and Global Accuracy (89.5%), when compared to any of the three techniques employed individually. In ROC curves analysis, combined BB + BW showed the highest diagnostic significance with an Area Under Curve (AUC)=.828 (p value = .030), followed by BB with an AUC=.774 (p value=.004) and FNA with an AUC = .767, (p value= .042). Combination of bronchial brush and bronchial wash complement each other and enhance the diagnostic efficacy of lung cytology for the diagnosis of primary lung carcinoma and are more superior when compared with bronchial brush, bronchial wash or FNA performed individually.
Keywords
Bronchial, lung, carcinoma, brush, fine needle, lung
Introduction
Lung cancer is currently the number one cause of cancerrelated deaths worldwide, with about 1.6 million deaths in 2012 [1,2]. In the United States, more than one-quarter of all cancer deaths are due to lung cancer alone in both women and men accounting for 26% and 28 % respectively[2].Reports from United States and China state that lung cancer has a high mortality rate and that mortality rate is usually ascribed to late diagnosis [3,4]. In Saudi Arabia, the prevalence of lung cancer has increased significantly in the recent years which has been mainly attributed to the increased incidence of cigarette smoking among men and women in the community. According to the reports of Saudi cancer registry 2010, there were 397 cases of lung cancer accounting for 4% of all diagnosed cancers in that year. Thus, lung cancer ranked fifth among male population malignancies and thirteenth among female population malignancies [5]. In a recent study from our center, lung cancer ranked as the first cause of hospitalization in patients with respiratory diseases accounting for 31.2% of total cases [6] which is in contrast to the previous study also conducted at our center ,where it ranked as the fourth cause [7]. This may be indicative of an increasing awareness among patients regarding early diagnosis or may be due to a true increase in frequency of lung cancer.
Worldwide carcinomas account for nearly 95% of all cases of lung cancer, whereas sarcomas and lymphomas account for most of the remainder [8]. Primary lung carcinomas (PLCs) are classified as Non Small Cell Lung Carcinoma and Small Cell Lung Carcinoma (NSCLC and SCLC) [8]. Accounting for 75-80% of all cases, NSCLC is the more common of the two types [9].
To address the high mortality associated with lung cancer successfully, it should be diagnosed at an earliest possible stage. For early diagnosis different diagnostic cytological modalities are available which include; brushing, washing and fine needle aspiration . Approximately 70% of lung cancers are un-resectable as patients present in advanced stages and so cytology specimens continue to remain the primary method of diagnosis for the majority of lung cancer patients [9].Cytology is a powerful tool in the diagnosis of lung cancer, particularly in the distinction of NSCLC and SCLC which confers therapeutic significance to it [10]. Both BB (bronchial brush ) and BW ( bronchial wash ) are very effective in the diagnosis and differential diagnosis of lung cancers. Bronchial brushings often offer excellent specimens and accurate morphology of the site of the lesion [11] . Fine needle aspiration (FNA) has the highest sensitivity for endobronchial malignant lesions [12] and has also been used as the gold standard diagnostic test. However FNA cannot be performed in more peripheral sites or in patients at risk of hemorrhage.
As such BB and BW used as alternative methods for obtaining diagnosis are sometimes required [13]. Disagreement persists regarding the value and reliability of BB and BW cytology in comparison with histology for the diagnosis of malignancy. It is not possible to perform all techniques in each patient because each has specific advantages and disadvantages and better diagnostic yield is often obtained when cytological techniques are used together with bronchial biopsy [14].
However, the question regarding which combination of cytological and histological procedures provides the optimum diagnostic yield has not been conclusively answered but probably depends on the expertise and resources available at any individual center.
Aim
This study aims to determine the diagnostic value of lung cytology specimens, such as Bronchial wash, Bronchial brushing and Fine needle aspiration in diagnosing primary lung carcinoma individually ; and compare them to diagnostic value of combined BB and BW specimens among patients attending King Abdulaziz University Hospital, Jeddah Saudi Arabia..
Materials and Methods
This is a retrospective study of all cytology specimens performed for a clinical diagnosis of primary lung cancer between Jan 2000-Dec 2013, in King Abdulaziz Univerisity Hospital (KAUH). Specimens were identified by a computerized search through the cytopathology archives of Anatomic Pathology department, for the study period. Among all primary lung cancers only primary lung carcinomas (PLCs) were targeted for this study. The cytological samples included both exfoliative type (bronchial washing and bronchial brushing) and fine needle aspirative type (transbronchial and transthoracic) .The cytology specimens were taken by clinicians, who were either pulmonologists; or radiologists in case of computerized tomography (CT) guided FNA cytology. The initial work up to diagnose lung cancer was either through performing a flexible fiberoptic bronchoscopy (FFB) and/or computed tomography (CT) guided biopsy. Open lung biopsy was performed if bronchoscopic cytologic specimens and/or CT guided lung lesional biopsy failed to obtain a diagnostic material.
BB was performed using re-usable brush with nylon bristles, which was cleaned carefully between procedures to enhance collection of satisfactory material for cytology. Once the tumor was brushed, brush was withdrawn and the material cells were transferred directly onto 5-6 clean glass slides. The bristles of the brush were pressed against the slide with the aid of pressure from a needle. Air drying was avoided. Slides were immersed in a jar filled with 95% ethyl alcohol for fixation as quickly as possible for Papanicolaou stain (PAP), Hematoxylin and eosin (H&E) and Diff quick (DQ) staining. Smears were prepared using sediments and stained by PAP, H&E and DQ. The remaining material was used for cell block preparation wherever possible.
Bronchial washings (BW) were collected after brushing samples and were obtained by lavage with 20-40 ml of normal saline, and subsequent aspiration into a trap connected to the suction tubing. If the tumor was visible, the tip of the bronchoscope was positioned next to the tumor and if the lesion was peripherally located then the tip was wedged into the area where lesion was located.
FNA cytology lung was performed using a FFB needle for centrally located lesion using a 22-guage needle (TBNA; trans-bronchial needle aspiration). If the lesion was peripherally located, the procedure was performed under a CT guidance (TTNA; trans thoracic needle aspiration). Rapid on-site evaluation comparable is routinely provided by a qualified and experienced cytopathology technologist to process and interpret the stained wet film of the aspirate, immediately, and report the adequacy result to the bronchoscopist or the radiologist. All cytological specimens were prepared according to the standard processing protocol in our laboratory .PAP stain was used for wet fixed smears and Diff Quick stain was used for air dried smears.
Cytology smears were reviewed by two pathologists separately, then jointly for more accuracy and diagnostic consensus. Cytology samples with inadequate cytological material were excluded. Cytological analysis was considered positive only when large numbers of definitely malignant cells were present.
For our study, we performed a computerized search through the histopathology archives of Anatomic Pathology department, using Systematized Nomenclature of Human Medicine (SNOWMED) morphologic codes for all patients diagnosed as PLC, including open and excisional lung biopsies. Initially, all patients with lung cancers were filtered then re-screened to include only those with both cytology and histology specimens for comparison. The included specimens (both cytological and histological) were reviewed by two pathologists to get a more concordant diagnosis. All biopsies were handled as per standard histopathological techniques which include paraffin embedding and Hematoxylin and Eosin (H&E) staining.
Results for various diagnostic outcomes were calculated on the basis of Cibas ES cytological diagnostic criterion [15].
Histological correlations
1. Positive cytological findings and those highly suspicious were considered as true positive (TP) when subsequent histological examination revealed a carcinoma.
2. Negative cytological findings were considered true negative (TN) when subsequent histological examination revealed a benign lesion.
3. The results of benign lesions on histology reported as highly suspicious on cytology were considered as false positive (FP).
4. The results of malignant lesions on histology reported as negative on cytology were considered as false negative (FN).
The procedures followed in the present study were in accordance with the ethical standards of the hospital ethical committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000.The PLCs were classified according to the most recent WHO (World Health Organization) classification of lung tumors [8]. Each category is classified in number, percentage, male to female ratio, and the age distribution. All cases were divided according to four specific age groups as follows: 20-39, 40-59, 60 -79, 80 and more years.
Statistical Methods
All statistical analyses were performed using Statistical Package for Social Sciences version 16.0 for Windows (SPSS Inc., Chicago, IL, USA). Descriptive statistics were used to obtain means +/- SD (standard deviations) and frequencies of the variables studied. To determine the diagnostic values of each cytological technique (BB, BW, FNA and BB+BW), respective findings were correlated with the histological diagnosis using either chi-square test; or Fisher’s exact test for analyses showing at least one sub-group with less than 5 observations. Thus, sensitivity (Sn), specificity (Sp), positive predictive value (PPV) and negative predictive value (NPV) were calculated for each technique, with respective significance levels. A two-tailed significance test with p-value of less than 0.05 was considered statistically significant.
Results
The computerized search through Anatomic Pathology archives for the study period found 199 cytological procedures for clinical diagnosis of PLC, belonging to 118 patients. Eighty five (72 %) patients had more than one cytological technique practiced. Regarding the frequencies of the cytology techniques used, 100 were BBs, 63 were BWs and 36 were FNAs. Histological correlations were available for all BW (63, 100%) and FNA (36, 100%) specimens, but only for 78 BBs out of the 100 (78%); giving a total of 177 cytological specimens for 98 patients included in the study. Ninety eight patients (83%) were diagnosed as PLC on cytology, which were confirmed on histological correlation. The most common age group for PLC was 60-79 years (n=58, 59.1%) followed by 40-59 years (n= 29, 29.5%). No patients were recorded in the age group below 20 years .Two patients (2%) were above 80 years of age. There was male predominance with overall male to female ratio of 4.4:1 (Table Ia).
Cytological findings
Regardless of the tumor type, 140 (79.1%) of the total 177 cytological specimens were interpreted as positive for PLC( that is the TP fraction in each category). These are distributed regarding specific technique as follows: 58/78 BBs (74.3%), 44/63 BWs (69.8%), 28/36 FNA (77.8%) and 49/57 combined BB and BW (85.9%).
With regards to tumor type, cytological techniques revealed 62 (63.2%) cases of NSCLC not otherwise specified type (NOS) with a male ratio of 3.4:1; followed by adenocarcinoma (AC) with 18 cases (18.3%) and female predominance; small cell lung carcinoma (SCLC) with 9 cases (9.1%), exclusively in males; squamous cell carcinoma (SQCC) with 8 cases only (8.1%) and male predominance and 1( 1%) adenosquamous carcinoma in a male patient (Table Ib). For the remaining 20 patients, diagnosis of PLC was ruled out.
Histological correlations
When correlated to respective histological findings, using cross-tabulation analysis, FNA showed the best Sn (86.7%), followed by BB (82.1%) and BW (74.5%) used individually. However, BB had the best Sp of the three cytological techniques, followed by FNA and then BW, respectively 72.7%, 66.7% and 62.5%. All of the three techniques had a very good PPV (>90%), but average or bad NPV (≤50%) (Table II). Combined analysis of BB and BW specimens revealed a higher diagnostic value, as compared to any of the three cytological technique analyzed individually; with a Sn= 90.6%, Sp = 75%, PPV = 98%, (p=.007). However, NPV of combined BB and BW (37.5%) was weaker than in FNA and BB but greater than BW, respectively 50.0%, 40.0% and 26.3% (Table II).
Besides Sn, Sp, PPV and NPV, the following parameters were also calculated to assess and compare the diagnostic accuracy of the three cytological techniques studied: False positive index (FPI)= FP/ FP+TN x 100 False negative index (FNI)= FN/ FN +TP x100 Global Accuracy (GA)= TP+TN/TP+TN+FP+FN x 100 Comparing respective results showed that combined BB and BW have the best indices, with the lowest FNI (9.5%) and FPI (25%) and the highest GA (89.5%), when compared to any of the three techniques employed individually (Table II) .Similarly, ROC curves analysis demonstrated that combined BB and BW had the highest diagnostic significance with an Area Under Curve (AUC) =.828 (p value = .030), followed by BB (AUC=.774, p value =.004) , FNA (AUC = .767, p value = .042) and BW (AUC =.685, p value = .092) (Figures 1a, 1b, 1c and 1d).
Regarding tumor type detection, combined BB and BW have allowed detecting 77.4% (48 out of 62) cases of NSCLC on cytology both being positive. The remaining 12 patients of NSCLC were diagnosed as AC and 8 as SQCC either by performing BB or FNA alone. BB and BW together were also useful in diagnosing 7 of 9 (78 %) patients with SCLC, while the remaining 2 SCLC were diagnosed by BB or FNA alone. Overall only 5 patients who had both BB and BW completely negative on cytology turned out to be positive on histology. Among these were 3 NSCLC, 1 AC and 1 SQCC.
Discussion
There is increasing awareness to render the most accurate diagnosis using the least invasive procedures. As such respiratory tract cytology has been well established throughout the world as a diagnostic procedure in the evaluation of patient with suspected lung malignancy [16]. Technologic advances in FFB continue to improve our ability to perform minimally invasive, accurate evaluations of the tracheobronchial tree and to perform an ever-increasing array of cost effective diagnostic interventions [16]. This is shifting the focus from diagnosis of advanced lung cancer in inoperable patients to the use of cytology as a first line diagnostic tool.
FFB is used to diagnose both central and peripheral lung lesions. It is the simplest method for obtaining material from the suspicious lesion with little morbidity and almost negligible mortality [17]. More than 70% of lung carcinomas are visible using the FFB and although the yield is dependent on operator’s experience, a high level of diagnostic accuracy can be achieved by taking between three and five specimens and a combination of brushing, biopsy and bronchial washes can push the accuracy to establish a diagnosis in 60% of cases [17,18]. The diagnostic yield for endobronchial biopsy when a lesion is visible is 70–90% [19].When the tumor is visible but is intramural rather than endobronchial in distribution, the diagnostic yield falls to 55% and is reduced further when the tumor lies beyond the bronchoscopes’ vision which would necessitate a CT guided fine needle biopsy of the lung [17]. The diagnostic yield of bronchoscopy decreases for peripheral lesions and depends on a number of factors, including lesion size, the distance of the lesion from the hilum and on the relationship between the lesion and bronchus. The yield of bronchoscopy for lesions ,less than 3 cm varies from 14–50% compared with a diagnostic yield of 46–80% when the lesion is more than 3 cm [16].
The accuracy in differentiating between SCLC and NSCLC cytology for the various cytological diagnostic modalities has been reportedly variable. In a recent study of 192 preoperative cytology diagnosis the accuracy was 93%, and for the definitive diagnoses it was 96%. The diagnostic sensitivity of BB in detection of lung malignancy varies between the studies from 48 to 85 % [20,21].This wide ranges can be explained by different techniques used to obtain the cytological specimens and the inclusion of suspicious cases as positive when calculating the sensitivity. At our center, the sensitivity of BB is high 82 % with overall accuracy of 80.7%. This could be explained partly by the fact that most of our patients present at advanced stage with easily visible tumor by bronchoscope.
The value of performing BW for diagnosing lung cancer is variable. Some studies suggest increase in the diagnostic yield by adding BW to BB and endo-bronchial biopsy [16]. Where others show no additional diagnostic value [22]. In a recent study of 503 patients by Liam et al [23] BW was the only procedure with a diagnostic yield in 7.3 % of their patients with bronchoscopically visible tumor. Bodh et al [24] found the overall Sn to be higher if both BB and BW are used together in the diagnosis of visible tumor. In our study 53 patients had both BB and BW performed and in these patients the Sn of the diagnosis increased by 25%.The Sn in our study was especially impressive when both BB and BW were combined to predict NSCLC versus SCLC however it was poor for subtyping AC versus SQCC. This can be explained by the fact that the cytological criteria that separate NSCLC and SCLC are strong and clear with no overlap while the heterogeneity within the NSCLC group limits more specific categorization on cytological examination. We however found no correlation to support the observations presented by Tuladhar et al. that BB was the most sensitive technique for diagnosis of SCLC (80%) followed by SQCC (35.7%) [21].
Fine needle aspiration (FNA) cytology is an easy and reliable technique for diagnosing lung masses. It can be done either TTNA or TBNA. It is the procedure of choice for sampling peripheral lung lesion with a diagnostic accuracy of 80–95% [25]. In endobronchial lung cancers TTNA is a safe diagnostic tool. Although the diagnostic success has increased in all localizations by the addition of TBNA to conventional diagnostic methods, statistically significant result has been obtained only for lesions located at trachea and the main bronchi. The sensitivity of TBNA was reported as 90% in a study from India [26]. Errors due to superficial necrosis of deeply situated lesions can be avoided using this technique.
The most common age group for PLC in our study was 60-79 years which is in concordance with the literature. There is male predominance with M: F ratio of 4.4:1 and this is most likely related to the higher incidence of smoking among males. The pattern is very similar to age and gender distribution worldwide. The most common cytological diagnosis at our center is NSCLC (NOS) (62 %) followed by AC (18%). Factors that contributed to the greatest difficulty in making a specific diagnosis included poor differentiation and low specimen cellularity. Our results are almost similar to two recent studies by DS Gaur et al [13] and Baviskar et al [26].
This study has certain limitations and the results should be interpreted keeping them in mind. The first being that the results of the present study were based on a small sample size which limits their application to a larger population. The second being that although 42.7% of patients had undergone more than one cytological procedure yet the number of patients having undergone all three procedures in a systematic fashion was very low further limiting our capacity to ensure more error free comparisons. Other limitations that might have been contributory could be inter observer variability of the cytopathologists and variability in the processing time of the cytological material. These variations could also explain the discordance between the results of different studies concerning the cytological examination yield and that of establishing a diagnosis of PLC through these techniques .
In conclusion, the high Sn and Sp for combined BB and BW obtained in this study indicates that they are a reliable diagnostic technique when performed together by the clinician and interpreted by an experienced cytopathologist . BB and BW specimens are complimentary in diagnosis of PLC. The combination appears to serve accurately in the distinction between NSCLC and SCLC.Individually FNA has the best diagnostic value in terms of a significantly superior Sn and GA among all of three cytological techniques. Diagnostic value of BB is superior in terms of PPV and Sp. Although variable degree of cytohistological discrepancies do occur, we try to emphasize the fact that all three cytological techniques studied are the least invasive and helpful in the diagnosis of PLC.
References
- Brambilla E, Travis WD. Lung cancer. In: Stewart BW, Wild CP (eds) World Cancer Report. World Health Organization, Lyon, 2014; 489-508
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA. Cancer J. Clin 2014; 64 ( 1): 9-29.
- Siegel R, Naishadham D, Jemal A. Cancer statistics 2012., CA. Cancer J. Clin 2012; 62 (1): 10-29.
- Chen W, Zhang S, Zou X. Estimation and projection of lung cancer incidence and mortality in China. Zhongguo Fei Ai Za Zhi 2010;13 (5): 488-93
- Al-Eid H, Quindo M, Cancer incidence report SaudiArabia 2010: 48
- Abdullah LS, Mufti ST. Histopathological Pattern of Respiratory Diseases among Patients attending a Tertiary Care Centre in Western Saudi Arabia from January 2000 - December 2010. Saudi Journal of Internal Medicine 2012; 2(2): 15-20.
- Alamoudi OS. Lung cancer at a University Hospital in Saudi Arabia: A four-year prospective study of clinical, pathological, radiological, bronchoscopic, and biochemical parameters. Ann. Thorac. Med 2010 : 5(1): 30-36
- Travis WD and Rekhtman N. Pathological diagnosis and classification of lung cancer in small biopsies and cytology: strategic management of tissue for molecular testing. Semin. Respir. Crit. Care Med 2011 ;32(1): 22-31
- Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, Garg K, Austin JH, Asamura H,Rusch VW, Hirsch FR, Scagliotti G, Mitsudomi T, Huber RM, Ishikawa Y, Jett J, Sanchez-Cespedes M, Sculier JP, Takahashi T, Tsuboi M, Vansteenkiste J,Wistuba I, Yang PC, Aberle D, Brambilla C, Flieder D, Franklin W, Gazdar A, Gould M, Hasleton P, Henderson D, Johnson B, Johnson D, Kerr K, Kuriyama K,Lee JS, Miller VA, Petersen I, Roggli V, Rosell R, Saijo N, Thunnissen E, Tsao M, Yankelewitz D. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol 2011; 6(2): 244-285.
- Rivera MP, Mehta AC. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007; 132 (3S ): 131S- 148S.
- Melamed MR.Tumors of the lung: Conventional cytology and aspiration biopsy, In Koss’ Diagnostic Cytology and Its Histopathologic Bases. 5 th ed, Lippincott Williams & Wilkins. 2006:643-712.
- Schreiber G, McCrory DC . Performance Characteristics of Different Modalities for Diagnosis of Suspected Lung Cancer : Summary of published evidence. Chest 2003; 123(1): 115S-128S.
- DS Gaur, M Harsh, S Kishore, S Kohli, A Kusum, VP Pathak . Efficacy of bronchial brushings and transbronchial needle aspiration in diagnosing carcinoma lung. J Cytol 2007; 24( 1): 46-50.
- Rivera MP, Mehta AC, Wahidi MM Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143(5): 42S-65S.
- Cibas ES. Laboratory management, In Cytology Diagnostic Principles and Clinical Correlates, 4th ed., Cibas ES and Ducatman BS, Eds. Elsevier Saunders Philadelphia, 2014: 538-545.
- Herth FJF. Bronchoscopic techniques in diagnosis and staging of lung cancer. Breathe 2011; 7 ( 4): 324-337.
- El-Bayoumi E and Silvestri GA. Bronchoscopy for the diagnosis and staging of lung cancer. Semin Respir Crit Care Med 2008; 29 ( 3): 261-270.
- Ofiara LM, Navasakulpong A, Beaudoin S, Gonzalez AV. Optimizing tissue sampling for the diagnosis, subtyping, and molecular analysis of lung cancer. Front. Oncol 2014; 4: 253.
- Mazzone P, Jain P, Arroliga AC, Matthay RA. Bronchoscopy and needle biopsy techniques for diagnosis and staging of lung cancer. Clin Chest Med 2002; 23 ( 1): 137-158.
- Rekhtman N, Brandt SM, Sigel CS, Friedlander MA, Riely GJ, Travis WD, Zakowski MF, Moreira AL. Suitability of thoracic cytology for new therapeutic paradigms in non-small cell lung carcinoma: high accuracy of tumor subtyping and feasibility of EGFR and KRAS molecular testing. J Thorac Oncol 2011; 6 ( 3): 451-458,
- Tuladhar A, Panth R, and Joshi A .Comparative analyses of cytohistologic techniques in diagnoses of lung lesions. J Pathol Nepal 2011; 1 ( 2): 126-130.
- Karahalli E, Yilmaz A, Türker H, and Özvaran K. Usefulness of Various Diagnostic Techniques during Fiberoptic Bronchoscopy for Endoscopically Visible Lung Cancer: Should Cytologic Examinations Be Performed Routinely? Respiration 2001; 68 ( 6): 611– 614.
- Liam CK, Pang YK, Poosparajah S. Diagnostic yield of flexible bronchoscopic procedures in lung cancer patients according to tumour location. Singapore Med J 2007; 48 (7): 625- 631.
- Bodh A, Kaushal V, Kashyap S, Gulati A. Cytohistological correlation in diagnosis of lung tumors by using fiberoptic bronchoscopy: study of 200 cases. Indian J Pathol Microbiol 2013; 56 ( 2) : 84-88.
- Ly A. Fine-needle aspiration biopsy techniques and specimen handling, In Cytology Diagnostic Principles and Clinical Correlates, 4th ed., Cibas ES and Ducatman BS, Eds. Elsevier Saunders Philadelphia, 2014: 221–230.
- Baviskar BP and Dongre SD. A Comparative Study of Bronchial Brushing and Transbronchial Needle Aspiration in Diagnosis of Lung Tumors. Glob. Res. Anal 2013; 2 ( 3): 156-157.