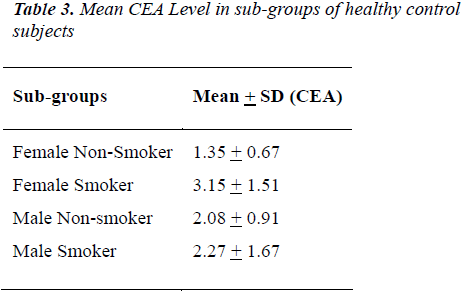- Biomedical Research (2013) Volume 24, Issue 3
Diagnostic precision of carcinoembryonic antigen level in esophageal carcinoma.
Bhawna Bagaria1*, Sadhna Sood1, Rameshwaram Sharma2 and Soniya Lalwani31SMS Medical College and Hospital, Jaipur, Rajasthan, India
2Department of Radiotherapy and Oncology, S.M.S. Medical College & Hospital, Jaipur (Rajasthan), India
3Soniya Lalwani (Statistician) Statistics, Advanced Bioinformatics Centre, Birla Institute of Scientific Research, Jaipur (Rajasthan), India.
- Corresponding Author:
- Bhawna Bagaria
Dept. of Biochemistry
S.M.S.Medical College, Jaipur 302004, India
E-mail: bhawna_bagaria@yahoo.com
Accepted date: April 26 2013
Citation: Bagaria B, Sood S, Sharma R, Lalwani S. Diagnostic precision of carcinoembryonic antigen level in esophageal carcinoma. Biomed Res- India 2013; 24 (3):353-358.
Abstract
Esophageal cancers linked to lifestyle and environmental factors appears to possess varying abilities to produce Carcinoembryonic Antigen (CEA), hence in this study the diagnostic precision of CEA in patients initially diagnosed of esophagus cancer before receiving any anticancer therapy was evaluated. Serum CEA levels were assessed in healthy subjects and patients initially diagnosed of esophageal carcinoma by endoscopic examination and biopsy (N=50 each), both categories including male and female smokers and non-smokers. The test was performed by two site sequential Chemiluminescent Immunometric assay kit. Sensitivity, Specificity, Positive Predictive Value (PPV) and Negative Predictive Value (NPV) of CEA was found out and Receiver Operating Characteristic (ROC) curve plotted. Serum CEA levels were significantly higher in esophagus cancer patients as compared to control and in healthy smokers as compared to non-smokers. The overall sensitivity and specificity of CEA for detecting esophagus cancer was 38% and 80% respectively. The AUC was 0.772 (SE=0.04) and significance level P<0.0001. PPV was 65.51% and NPV was 56.34%. Using ROC curve analysis the optimum CEA cut-off point for the best combination of sensitivity 66% and specificity 86% was > 2.81 ng/ml. The CEA positivity rate before therapy was 38% which dropped to 18% after therapy. Serum CEA is a test with high specificity but insufficient sensitivity for detecting esophageal cancer in isolation. However, it may help as an additional parameter in screening the risk groups and monitoring of palliative therapy.
Keywords
CEA – Esophagus Cancer - NPV- PPV – ROC curve –Sensitivity - Specificity.
Introduction
The annual incidence rate of esophageal cancer is increasing having mortality sixth in malignant tumor. This cancer has very poor response to current means of therapy and have devastating prognosis. The five year survival rate of esophageal cancer detected early is 70% after surgical treatment, while in patients with advanced cancer the survival rate is just 10-13% [1]. Thus management of patients with esophageal cancer is a continuing challenge for the surgeons [2]. Currently, esophagus cancer is treated by surgery with chemo and radiotherapy. The anatomic features of this organ impose radiotherapeutic and surgical limitations which contribute to poor endresults. These facts assume great significance when one realizes that cancer of the esophagus probably has high incidence in India seen at the Tata Memorial Hospital, Bombay and even in our state at S.M.S. Hospital, Jaipur. Thus, the treatment of Esophagus cancer whether by surgery or chemo and radiotherapy, needs highly sensitive diagnostic methods. Studies have supported pre-operative & post-operative measurements of serum CEA in the diagnosis, prediction of prognosis & monitoring recurrence in patients with esophageal carcinoma [3-8].
CEA described by Gold and Freedman in 1965 is a b1– glycoprotein with a molecular weight of approx. two lakh located on the luminal surface of tumor cell membrane. It is normally found in gastrointestinal tract of embryos and in smaller concentration in normal adult tissue. It functions as an adhesion molecule promoting the aggregation of human gastrointestinal cancer cells [9]. Subsequent studies have shown that concentration of CEA in malignant tissue is assessed 60 fold higher than in nonmalignant tissue [10]. CEA testing is of significant value in the monitoring of patients with diagnosed malignancies in whom changing concentrations of CEA are observed. A persistent elevation in circulating CEA following treatment is strongly indicative of occult metastatic and / or residual disease. A persistently rising CEA value may be associated with progressive malignant disease and a poor therapeutic response. A declining CEA value is generally indicative of a favorable prognosis and a good response to treatment. Patients who have low pre therapy CEA levels may later show elevations in the CEA level as an indication of progressive disease.
Therefore, this study was planned under following aims: 1) To evaluate the diagnostic precision of serum CEA in patients suffering from esophageal cancer before receiving any sort of therapy and diagnosed recently. 2) To calculate the Sensitivity, Specificity, PPV and NPV of CEA in detection of esophageal cancer. 3) To find out best cut-off point of CEA for optimum combination of sensitivity and specificity by ROC curve analysis so that it may serve as an additional survelliance investigation for diagnosing and timely institution of palliative therapy among esophagus cancer patients and may even help to screen the risk groups in healthy smokers.
Material and Method
Study Subjects
The subjects included 50 patients suffering from esophageal cancer diagnosed by endoscopic examination and biopsy and have not received any anticancer therapy before. 50 healthy subjects devoid of cancer were taken as normal control group.
All patients and healthy control subjects were recruited from Radiotherapy Department, S.M.S. Hospital, Jaipur from July 2011-June 2012.
Inclusion criteria
Healthy subjects: Both smoker and non smoker not diagnosed of any gastrointestinal disease or cancer.
Patients: Those suffering from esophagus cancer currently diagnosed by endoscopic examination and biopsy and have not received any anticancer therapy.
Exclusion criteria
Healthy subjects with any sort of GI infections or disease. For cancer patients: Patients receiving therapy from long time.
Study design
Clinical history
Each patient was strictly examined first by brief clinical history related to diet, lifestyle, initial symptoms or any treatment received before.
Smoking History
For every subject a brief smoking history was taken, whether smoker or non-smoker, method of smoking via cigarette, pipe, cigar, tenduleaf smoking stick etc. Quantity smoked per day if more than 10 cigarettes per day than heavy smoker, less than that smokers, and who did not smoke, or quit smoking from last few months were under non-smokers.
The patients and healthy subjects were categorized into: A. Esophageal cancer patients. B. Healthy subjects taken as control.
Both the categories i.e. of esophagus cancer patients and healthy control subjects were further divided under various sub-groups:
1. Female non-smoker
2. Female smoker
3. Male non-smoker and
4. Male Smoker.
A written and informed consent was obtained from all the study subjects. The present study was performed after the approval of institutional research committee, S.M.S. Medical College and Hospital, (Rajasthan univ. of health sciences) Jaipur, Rajasthan, India. Ref.no.RS/278.
Sample Collection
The Blood samples were collected before starting of any therapy in esophageal cancer patients and as a part of routine investigation in healthy subjects. Samples were taken in plain vial and allowed to clot. Serum was separated by centrifuging at 3000 rpm for 10 min and stored at – 20oc till further assay was performed.
CEA Measurement
CEA levels were determined by Commercial Immulite- 2000 a solid phase, two site sequential Chemiluminescent Immunometric assay kit (Immulite-2000, Siemens. Llanberis, Gwynedd, U.K.) Reference no. L2KCE2 according to the manufacturer’s instructions.
Taking into consideration the range for normal healthy control group the upper limit of carcinoembryonic antigen is:
• Female non- smoker: 2.5 ng/ml
• Female smoker: 4.9 ng/ml
• Male non-smoker: 3.4 ng/ml
• Male smoker: 6.2 ng/ml
(ng=nanogram, ml=millilitre )
CEA levels greater than upper limit for female nonsmoker and smoker and male non-smoker and smoker were considered elevated and below the upper limit of normal were considered non elevated.
Results
Data analysis
The data were analyzed using SPSS Software version 10.0 (SPSS Inc. USA) and MedCalc to estimate the significance of the observed differences and plotting ROC curves. Sensitivity, Specificity, PPV and NPV were calculated by the formulae given below and ROC curve plotted to give optimum cut-off of CEA for maximum sensitivity and specificity.
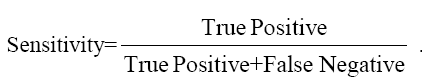 ....(1)
....(1)
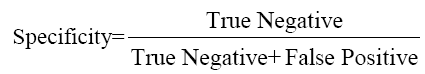 ....(2)
....(2)
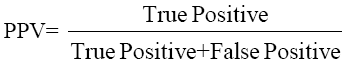 ....(3)
....(3)
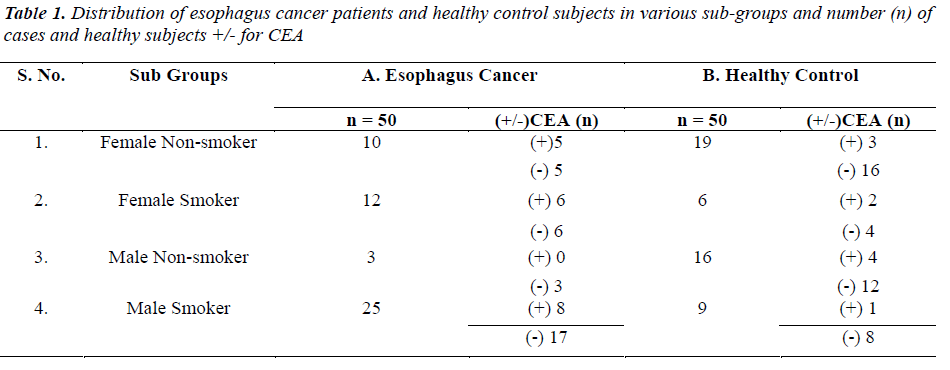
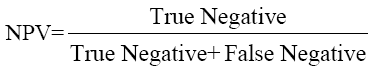 ....(4)
....(4)
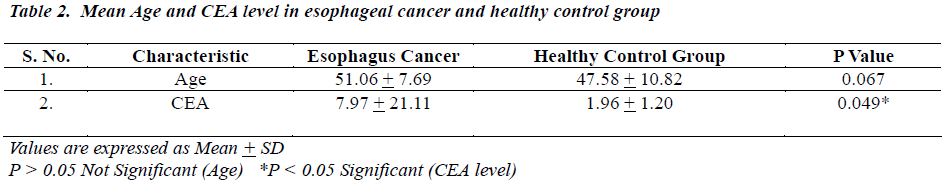
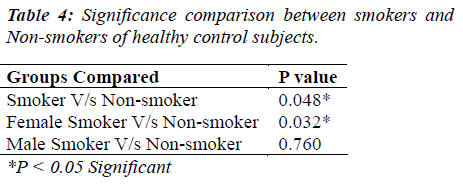
The distribution of 50 esophagus cancer patients and 50 healthy subjects classified as smokers and non-smokers and showing positive or negative result for CEA according to their specified range are given in Table 1. The positivity rate of CEA in esophagus cancer patients before therapy was 38% which dropped to 18% after receiving therapy (chemo/radio/surgery). The Mean + SD of Age and CEA in esophagus cancer patients and healthy control subjects are given in Table 2. There was no significant difference (P>0.05) seen in age (51.06 + 7.69 v/s 47.58 + 10.82) whereas the mean CEA level indicate significant difference (P<0.05) between esophagus cancer patients and healthy control (7.97 + 21.11 v/s 1.96 + 1.20). Table 3&4 gives mean CEA levels in healthy subjects in various sub-groups indicating a statistically significant difference of CEA level between smokers and non-smokers (P<0.05) not diagnosed of cancer presently. The sensitivity, specificity, PPV and NPV of CEA in detection of esophageal cancer given in Table 5 is as follows: the overall sensitivity and specificity of CEA for detecting esophageal cancer is 38% (95% CI: 19.5 – 46.7) and 80% (95% CI: 66.3 – 90.0) respectively. The PPV is 65.51% and NPV 56.34%. Fig.1 gives area under ROC curve 0.772, SE=0.0497 (95% CI: 0.678-0.850) and significance level P<0.0001 in esophagus cancer patients v/s control. The best cut off point of CEA for maximum combination of sensitivity 66% (95% CI: 51.2 – 78.8) and specificity 86% (95% CI: 73.3 – 94.2) estimated by ROC curve analysis is > 2.81 ng/ml.
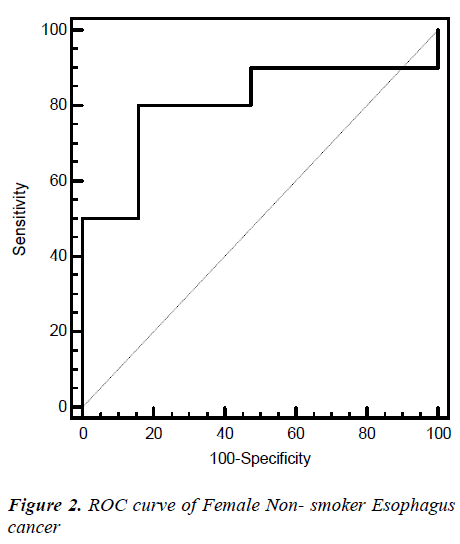
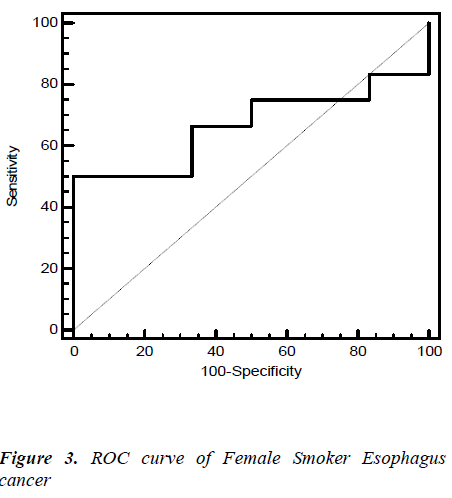
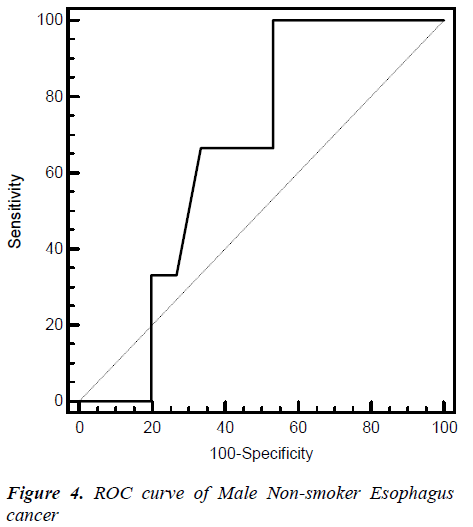
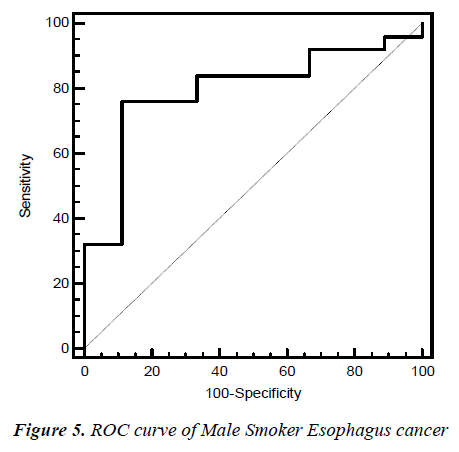
Fig. 2, 3, 4, 5 give the diagnostic precision of CEA in female non-smoker and smoker and male non-smoker and smoker suffering from esophagus cancer respectively. In Fig.2 female non-smoker our range >=2.5 is assume to be diseased. The AUC is 0.805 (SE=0.106) Significance level P 0.0041 (95% CI: 0.617-0.928). The optimum cut off point of CEA for maximum sensitivity 80% (95% CI: 44.4-97.5) and specificity 84% (95% CI: 60.4-96.6) detected by ROC curve is >1.8. In Fig 3.female smoker our range >=4.9 is assume to be diseased. The AUC is 0.667 (SE=0.131) Significance level P 0.2029 (95% CI: 0.410 - 0.867).The optimum cut off point for maximum sensitivity 50% (95% CI: 21.1-78.9) and specificity 100% (95% CI: 54.1-100) detected by ROC curve is >4.96. In Fig 4.Male non-smoker our range >=3.4 is assume to be diseased. The AUC is 0.615 (SE=0.140) significance level P 0.4133(95% CI: 0.368-0.825). The optimum cut off for maximum sensitivity 100% (95% CI: 29.2-100) and specificity 43% (95% CI: 19.8-70.1) detected by ROC curve is >1.73. In Fig.5 Male smoker our range >=6.2 is assume to be diseased. The AUC is 0.796 (SE=0.0859) significance level P 0.0006 (95% CI: 0.623-0.914). The optimum cut off point of CEA for maximum sensitivity 76% (95% CI: 54.9-90.6) and specificity 88% (95% CI: 51.8-99.7) detected by ROC is >2.8.
Discussions
A study published in Dec. 2011 estimated that in U.K. 89% esophageal cancers are linked to life style and environmental factors. 63% esophageal cancer in men and 71% in women in U.K. in 2010 were caused by smoking [11]. Thus, smoking proves to be a major risk factor for esophagus cancer as it causes acid reflux and damages cell DNA of esophagus [12]. Carcinoma of esophagus appears to possess varying abilities to produce CEA hence CEA levels appear to be promising as a marker of tumor presence and this is seen in our result also where esophagus cancer group has significant higher level of CEA as compared to control group [1]. This was even supported by studies of Eva Munck wikland et al [5]. Thus CEA may help along with other investigations in planning of palliative therapy and its monitoring. Our data also indicates significant higher levels of CEA in smokers as compared to non-smokers of normal control group which supports the view that CEA protein in serum of cigarette smoker is twice higher than non-smokers. This was even studied by I. Fukuda et al., Kornek G. et al [13,16]. Thus, in control group this may help to screen the risk groups for esophageal cancer, as smoking increases the risk of both squamous cell carcinoma and adenocarcinoma discussed with a recent pooled analysis of European studies showing a four fold increase of esophageal cancer overall among current smokers [14].The sensitivity and specificity of CEA in detecting esophageal cancer was near about the study of Anbreen et al. done in upper gastrointestinal tract carcinoma [15].
It is as yet an unrecognised fact in geographic pathology that cancer of the esophagus has high incidence in India. An analysis of patient material reveals that 51% of cases are too advanced for any treatment at their initial presentation. Carcinoma of esophagus is a very lethal disease relatively unresponsive to therapy. Thus a comprehensive diagnostic programme is of great importance for successful therapy.
A persistently rising CEA value may be associated with progressive malignant disease and a poor therapeutic response. A declining CEA value is generally indicative of a favorable prognosis and a good response to treatment. Patients who have low pre therapy CEA levels may later show elevations in the CEA level as an indication of progressive disease [17].
Our findings suggest that although, sensitivity of CEA is low, it may prove as an additional parameter in conjunction with clinical radiological and histological confirmation for diagnosing and timely institution of palliative therapy among esophagus cancer patients. The best cut-off value for optimum balance of sensitivity and specificity estimated by ROC curve may prove useful as an additional surveillance investigation for malignant v/s non-malignant disease.
Acknowledgment
We express our sincere thanks to the Cancer Radiotherapy Department, S.M.S. Hospital, Jaipur for providing their help in above study.
References
- Clinical significance of monitoring pre-operative CEA, CA 125, CA 19-9 and β-HCG serum level in esophageal cancer. Cancer Research > Esophagus Cancer > 2011.08.25. Available from: http://www.cancer-res.com/esophagus-cancer/.
- Geoffrey WB et al. Carcinoembryonic Antigen measurements in the management of esophageal cancer: An indicator of subclinical recurrence. Am J Surg. 1995; 170:597-601.
- Mao YS, Zhang DC, Zhaq XH, Wang LJ, Oi J, LiXX. Significance of CEA, SCC and Cyfra21-1 serum test in esophageal cancer. Zhonghua Zhong Liu Za Zhi. 2003; 25(5):457-60.
- Song K, Udagawa H, Aoyama N, Muro K. Treatment process and tumor marker of esophageal cancer. Esophagus 2009; 6: 137-142.
- Munck-Wikland E, Kuylenstierna R, Wahren B, Lindholm J, Haglund S. Tumor Markers Carcinoembryonic Antigen, CA 50 and CA 19-9 and Squamous Cell Carcinoma of the esophagus. Cancer 1988; 62: 2281-2286.
- Shin-ichi KosugiS, Tadashi Nishimaki, Tatsuo Kanda, Satoru Nakagawa, Manalu Ohashi, Katsuyoshi Hatakeyama. Clinical significance of serum Carcinoembryonic Antigen, Carbohydrate Antigen 19-9 and Squamous Cell Carcinoma Antigen levels in esophageal cancer patients. World J. Surg. 2004; 28:680-685.
- K Sakamoto, Y Haga, R Yoshimura, H Egami, Y Yokoyama, M Akagi. Comparative effectiveness of the tumor diagnostics, CA 19-9, CA 125 and Carcinoembryonic Antigen in patients with diseases of the digestive system. Gut 1987; 28:323-329.
- Kim YH, Ajani JA, Qta DM, Lynch P, Roth JA. Value of serial carcinoembryonic antigen levels in patients with resectable adenocarcinoma of the esophagus and stomach. Cancer 1995; 75(2):451-6.
- Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell 1989; 57:327-34.
- Boucher D, Cournoyer D, Stanners CP, Fuks A. Studies on the control of gene expression of the carcinoembryonic antigen family in human tissue. Cancer Res 1989; 49:847-52.
- Parkin DM, Boyd L, Walker LC. The fraction of cancer attributable to life style and environmental factors in the UK in 2010. Summary and Conclusions. Br. J Cancer 2011; 105(S2):S77-S81.
- Lisa Fayed. What is Esophageal Cancer? The causes symptoms, treatment and prevention of esophageal cancer. About. Com>Cancer> Esophagus Cancer>2008.¬ 09.04.Available from: http://cancer.about.com/od/esophagealcancer/Esophage al_Cancer.htm
- I. Fukuda, M. Yamakado, H. Kiyose. Influence of smoking on serum carcinoembryonic antigen levels in subjects who underwent multiphasic health testing and services. Journal of Medical Systems 1998; 22(2):89-93.
- Yuan-Chin Amy Lee, Manuela Marron, Simone Benhamou, Christine Bouchardy, Wolfgang Ahrens, Hermann Pohlabeln, etal. Active and involuntary tobacco smoking and upper aero digestive tract cancer risks in a multicenter case-control study. Cancer Epidemiol Biomarkers Prev. 2009; 18(12):3353-61.
- Anbreen M. Choudhary, M. Zameeer Ahmed and Mazhar S. Choudhary. Comparison of CEA and CA 19- 9 with CA 72-4 in patients with upper gastrointestinal carcinomas in local population. Biomedica 2010; 26:16-19.
- Kornek G, Depisch D, Temsch EM, Scheithauer W. Comparative analysis of cancer-associated antigen CA-195, CA 19-9 and carcinoembryonic antigen in the diagnosis, follow-up and monitoring of response to chemotherapy in patients with gastrointestinal cancer. J Cancer Res Clin oncol. 1991; 117(5):493-496.
- Malati T. Tumour Markers: An Overview. Indian Journal of Clinical Biochemistry. 2007; 22(2):17-31.