Keywords |
| Procalcitonin, Bacterial sepsis, Blood culture |
Introduction |
| Bacterial sepsis is associated with high morbidity and
mortality. Sepsis is the second most common cause of
death after myocardial infarction in patients admitted to
intensive care units. Mortality due to sepsis is as high as
25-35% and is higher in patients with septic shock [1].
Early diagnosis is critical in management of patients
with bacterial sepsis since appropriate antibiotics can be
initiated immediately. Blood culture remains the gold
standard for diagnosis of bacterial sepsis. However a major
limitation is unavailability of results within 48 hrs. Prior
antibiotic therapy often results in a negative blood culture
there by negating a confirmatory diagnosis of sepsis. Skin
colonizers often confound a culture outcome in patients
presenting with fever due to non-bacterial causes, resulting
in unnecessary antibiotic therapy. Several clinical markers of infection have been recommended but most are found
to be non-specific since they can be positive in systemic
inflammation of non-infectious origin. Hematological
markers of infection like total and differential counts may
also be non-specific [2,3,4]. |
| There is a continuous search for biomarkers of sepsis. Some
of the biomarkers that have been evaluated include lactate,
interleukins, C reactive protein (CRP) and procalcitonin
[4,5,6]. C reactive protein is most widely used acute
phase reactant and a sensitive marker of inflammation.
It cannot differentiate bacterial sepsis from other causes
of inflammation. CRP gets elevated only 24 to 48 h after
the infection is initiated, hence cannot be a rapid indicator
[7,8]. Reports of Procalcitonin (PCT) include its role in
diagnosis of bacterial sepsis, determining the severity
of sepsis and in determining the duration of antibiotic administration in children and adults [2,9]. This study was
designed to determine procalcitonin levels in patients with
sepsis and its correlation with blood culture outcome. |
Materials and Methods |
| The study was a retrospective study. 136 adult patients
(>18 years of age) admitted to a tertiary care hospital
between November 2012 to May 2014 were included
in the study. The sample size was calculated by taking
into account the prevalence of suspected cases of sepsis,
sensitivity and specificity of Procalcitonin (PCT). The
study was approved by the institutional ethical committee. |
Materials |
| Data on clinical features, laboratory investigations, blood
culture and PCT values were retrieved from the medical
records department of the institute. A diagnosis of sepsis
was made based on the recommendations of the American
College of Chest Physicians (ACCP) which included
presence of any of the following -2 or more of the features
along with suspected or proven source of infection:
Temp >38°C (100.4°F) or <36°C (96.8°F), Heart Rate
>90, Respiratory Rate >20 or PaCO2 <32 mmHg, WBC
>12,000/mm3, <4,000/mm3, or >10% bands. Patients with
cardiogenic shock, small cell lung carcinoma, medullary
carcinoma of thyroid, major trauma, major surgical
intervention, severe burns were excluded from the study,
as PCT is nonspecifically elevated in these conditions. |
Methods |
| Blood culture of all 136 patients was done by automated
BacT/Alert system. Procalcitonin was measured in Roche
e411 Electrochemiluminescence (ECLIA) automated
analyzer using PCT kit from B.R.A.H.M.S Diagnostica,
Berlin, Germany. According to the manufacturers, a value
of PCT >0.5 ng/ml was taken as pathological, 0.5 to 2 ng/
ml indicated that systemic infection could not be ruled out,
2 to 10 ng/ml indicated greater chances of sepsis and a value
of PCT above 10 ng/ml indicated severe bacterial sepsis. |
Statistical Analysis |
| Validation of PCT was done by calculating the sensitivity, specificity, positive predictive value, negative predictive
value. The cut off value of PCT which gave maximum
sensitivity and specificity were calculated by Receiver
operator curve (ROC) analysis. |
Results |
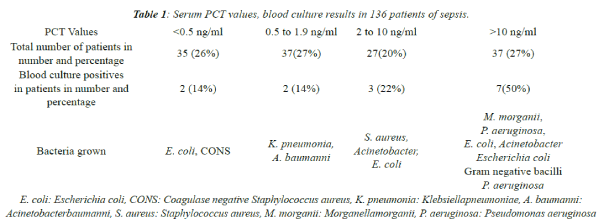
| Blood culture positivity and the bacteria isolated in
different ranges of PCT values are given in Table 1. The
sensitivity, specificity, positive predictive value (PPV),
negative predictive value (NPV) of PCT by taking a cut
off of 0.5 ng/ml or more as positive for sepsis are given in
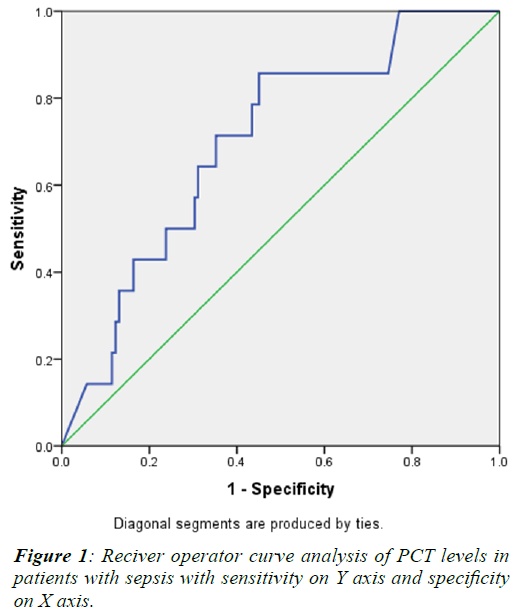
Table 2. Receiver operator curve (ROC) analysis was done
to determine the cut off value of PCT for which maximum
sensitivity and specificity was obtained. ROC analysis
is illustrated in Figure 1 which indicates a maximum
sensitivity of 64% and specificity of 65% at PCT value of
3.25 ng/ml. |
 |
 |
 |
Discussion |
| PCT is the precursor for the hormone calcitonin.
Calcitonin has a metabolic role in calcium homeostasis.
Though PCT is synthesized in the thyroid and pulmonary
cells in normal conditions, all tissues throughout the body
have the potential to express PCT [10]. Procalcitonin has
been studied as a diagnostic marker in many conditions
like fever of unknown origin, meningitis, respiratory tract
infections, urinary tract infections, burns and blood stream
infections. PCT has been studied as prognostic marker
with high values indicating bacterial load and severity of
sepsis. The concept of PCT clearance has been used as an
indicator of recovery [11]. |
| In the present study, by taking a cut off of >0.5 ng/ml as
an indicator of sepsis, the sensitivity, specificity, PPV and
NPV was found to be 85.7%, 25.4%, 11.7% and 93.9%
(Table 2) and by taking a cut off of >2 ng/ml, the sensitivity,
specificity, PPV and NPV was found to be 71.4%, 56.6%,
15.9% and 94.5% . Sinha M et al., observed a sensitivity
of 90% and specificity of 84% for a cut off of 0.5 ng/ml
where as a sensitivity of 85.7% and specificity of 94.7%
was noted with cut off of 2 ng/ml. They concluded that
PCT assay may avoid unwarranted antibiotic usage [12].
Harbarth et.al., observed a sensitivity of 97% and specificity
of 78% when a cut off of 1.1 ng/ml was used to diagnose
systemic inflammatory response syndrome [SIRS] with a
conclusion that PCT appeared to be a promising indicator
of sepsis in newly admitted, critically ill patients and PCT
values complemented with the clinical signs and routine
laboratory parameters, suggestive of severe infection [5].
Sudhir U et al., observed that a sensitivity of 94% had a
significant association between serum PCT and Sequential
Organ Failure Assessment (SOFA) score [13]. |
| Out of the 136 cases, 90 were males and 46 were females.
The mean PCT values in males were 15.9 ng/ml and
the mean PCT values in females were 13.9 ng/ml. The
difference was not statistically significant. Out of the 136
cases, 14 patients had a positive blood culture. Commonest
bacteria grown were Escherichia coli in 4 out of 14 culture
positive cases (Table 1 and Figure 2). Sucilathangam G
et al., observed Acinetobacter as the commonest organism
grown in 5 out of 14 culture positive cases [14]. Endotoxins
present in the bacterial cell wall induce the production of
PCT from the parenchymal tissues. The defense response
to infection also contributes to increase in PCT levels in
bacterial infections. The parenchymal cells do not have
the ability to convert PCT to calcitonin leading on to its
increased levels in circulation [16,17]. Sinha M et al.,
observed gram positive cocci as the commonest isolates
[12]. Findings of Wang H et al., showed Escherichia coli to be predominant isolate among gram negative organisms
similar to the present study. Coagulase-Negative
Staphylococcus and Enterococcus were the common gram
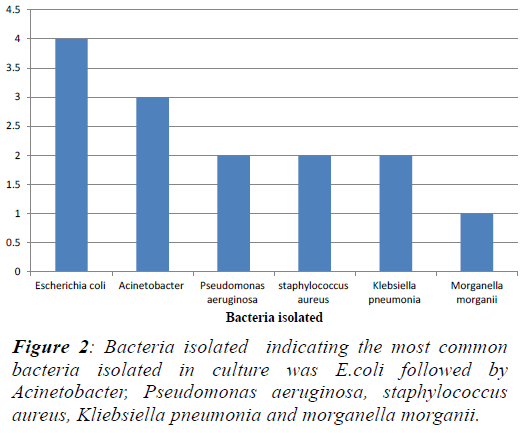
positive isolates [18]. In the present study, we observed
that out of 14 isolates, 12 were gram negative and only
2 isolates were gram positive. A possible correlation
between gram negative endotoxins and PCT levels needs
to be explored. The mean PCT values of patients with
gram positive isolates and gram negative isolates were 2.9
ng/ml and 31 ng/ml but the difference in the values in the
two groups were not statistically significant. (Table 3) |
 |
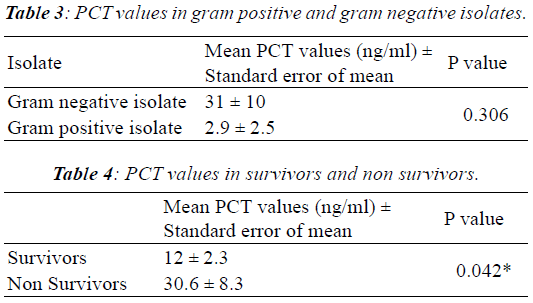
| The mean PCT value of those who succumbed to the infection
(23 in number) was 30.6 ng/ml whereas the mean value
in survivors was 12 ng/ml (Table 4). Our findings suggest
that high PCT values can indicate high mortality rate when
compared to patients with lower values, a finding similar
to Jensen J.U et.al., who observed that high PCT levels is
an independent predictor of mortality [19]. On the other
hand, Ruiz-Alvarez et.al, observed that PCT did not predict
mortality whereas CRP did [20]. Pettila et al., observed that
there was statistically significant difference in the PCT values
measured on first and second days in patients who survived
than patients who did not survive [21]. |
 |
 |
| It was observed that the mean PCT values of patients (80
in number) who were hospitalized for less than 10 days
was 15.7 ng/ml whereas mean PCT values of patients
hospitalized for more than 10 days was 14.5 ng/ml (Table
5). Two patients were culture positive but had PCT values
within normal range. The isolate from blood of one of
the patients was CONS, a normal skin commensal which
might not have induced the production of PCT. In another
case, a bacterium grown was E. coli and PCT was negative.
The reason could have been initiation of empiric antibiotic
prior to blood culture. It was observed that as the level
of PCT increased, the chances of blood culture positivity
increased. |
Conclusion |
| Bacterial sepsis is often a medical emergency requiring
intensive management with specific antibiotic therapy
and other supportive measures. A delay in detection of
sepsis may lead to poor outcome. Rapid sepsis markers
in the blood will help in overcoming the limitations of
confirmation by blood culture. Procalcitonin levels can
play a major role in not only detecting sepsis but also to
monitor progress or predict outcome. |
References |
- Chaudhury A, Rao TV. Bacteraemia in a tertiary care urban hospital in south India. Indian J PatholMicrobiol 1999; 42: 317-320.
- Castelli GP, Pognani C, Meisner M, Stuani A, Bellomi D, Sgarbi L. Procalcitonin and C reactive protein during systemic inflammatory response syndrome, sepsis and organ dysfunction. Crit Care 2004; 8: 234-242.
- Muller B, Schuetz P, Trampuz A. Circulating biomarkers as surrogates for bloodstream infections. Int J Antimicrob Agents 2007; 30: S16-23.
- Muller B, Becker KL, Schachinger H, RickenbacherPR, Huber PR, Zimmerli. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med 2000; 28: 977-983.
- Harbarth S, Holeokova K, Froidevaux C, Pittet D, Ricou B, Grau GE. Diagnostic value of procalcitonin, interleukin 6 and interleukin 8 in critically ill patients admitted with suspected sepsis. Am J RespirCrit Care Med 2001;164: 396-402.
- Nanda SK and Suresh DR. Plasma Lactate as prognostic marker of septic shock with acute respiratory distress syndrome. Indian Journal of Clinical Biochemistry 2009; 24: 433-435.
- PCNg. Diagnostic markers of infection in neonates. Arch Dis Child Fetal Neonatal 2004; 89: F229-F235.
- Weinschenk NP, Farina A, Bianchi DW. Premature infants respond to early-onset and late-onset sepsis with leukocyte activation. J Pediatr 2000; 137: 345-350.
- Ali AM, Walid FE, Abdelaziz SS. Procalcitonin Versus C-Reactive Protein in Neonatal Sepsis. J Immunol Infect Dis 2014; 1: 1-5.
- Kenneth LB, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. British Journal of Pharmacology 2010; 159: 253-264.
- Mehanic S, Baljic S.The Importance of Serum Procalcitonin in Diagnosis and Treatment of Serious Bacterial Infections and Sepsis. Mater Socio med 2013; 25: 277-281.
- Sinha M, Desai S, Mantri S, Kulkarni A. Procalcitonin as an adjuvant marker in sepsis. Indian Journal of Anaesthesia 2011; 55: 266-270.
- Sudhir U, Venkatachalaiah RK, Kumar TA, Rao MY, Kempegowda P. Significance of serum procalcitonin in sepsis. Indian J Crit Care Med 2011; 15: 1-5.
- Sucilathangam G, Amuthavalli K, Velvizhi G, Ashihabegum M.A, Jeyamurugan T, Palaniappan N. Early Diagnostic Markers for Neonatal Sepsis: Comparing Procalcitonin (PCT) and C-reactive protein (CRP). Journal of Clinical and Diagnostic Research 2012; 6: 627-631.
- Riedel S, Melendez JH, An AT, Rosenbaum JE, ZenilmanJM. Procalcitonin as a Marker for the Detection of Bacteremia and Sepsis in the Emergency Department. Am J ClinPathol2011; 135: 182-189.
- Linscheid P, Seboek D, Schaer DJ, Zulewski H, Keller U. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit Care Med 2004; 32: 1715-1721.
- Becker KL, Nylén ES, White JC, Müller B, Snider RH. Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J ClinEndocrinolMetab 2004; 89: 1512-1525.
- Wang H, Yin F, Shen DX, Zhang YJ, Luo YP, Liu CJ. Predictive value of procalcitonin for excluding bloodstream infection: Results of a retrospective study and utility of a rapid, quantitative test for procalcitonin. Journal of International Medical Research 2013; 41: 1671-1681.
- Jensen JU, Heslet L, Jensen TH, Espersen K, Steffensen P, Tvede M. Procalcitonin increases in early identification of critically ill patients at high risk of mortality. Crit Care Med 2006; 34: 2596-2602.
- Ruiz-Alvarez MJ, García-Valdecasas S, De Pablo R. Diagnostic efficacy and prognostic value of serum procalcitonin concentration in patients with suspected sepsis. J Intensive Care Med 2009; 24: 63-71.
- Pettila V, Hynninen M, Takkunen O. Predictive value of procalcitonin and interleukin-6 in critically ill patients with suspected sepsis. Intensive Care Med 2002; 28: 1220-1225.
|





