Research Article - Biomedical Research (2017) Volume 28, Issue 14
Diagnostic accuracy of plasma neutrophil gelatinase-associated lipocalin (NGAL) as an inflammatory biomarker for low-grade inflammation
Jong Weon Choi1*, Tatsuyoshi Fujii2 and Noriyoshi Fujii21Department of Laboratory Medicine, College of Medicine, Inha University, 27 Inhang-ro, Jung-gu, Incheon 22332, Republic of Korea
2School of Medicine, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8577, Japan
- *Corresponding Author:
- Jong Weon Choi
Department of Laboratory Medicine
Inha University, Republic of Korea
Accepted date: June 20, 2017
Abstract
Neutrophil gelatinase-associated lipocalin (NGAL) is an acute phase reactant in certain inflammatory conditions. The aim of this study was to investigate whether plasma NGAL is a sensitive biomarker of low-grade inflammation. The severity of inflammation was determined by a scoring system using highsensitivity C-reactive protein (hsCRP) and corrected erythrocyte sedimentation rate. The sensitivity of NGAL in low-grade inflammation was 27.3%, lower than hsCRP (73.8%, P<0.001). Plasma NGAL concentration was significantly associated with hsCRP, tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) in high-grade inflammation, but not in low-grade inflammation. In a receiver operating characteristic curve analysis, the diagnostic value of NGAL to identify an increase in IL-6 and TNF-α was similar to that of hsCRP in high-grade inflammation. However, the area under the curve of NGAL was significantly lower than that of hsCRP in low-grade inflammation (0.579 (95% CI, 0.449-0.708) versus 0.691 (95% CI, 0.557-0.826), P<0.001). Plasma NGAL may provide helpful information when monitoring patients with high-grade inflammation but does not seem to accurately reflect the severity of inflammation in low-grade inflammation.
Keywords
Neutrophil gelatinase-associated lipocalin, Tumor necrosis factor-alpha, Interleukin-6, Sensitivity, Inflammatory biomarker, Low-grade inflammation.
Introduction
Inflammation is an adaptive response to infection, noxious stimuli, and cellular injury that initiates elimination of toxic and foreign agents and repair of damaged tissue. Inflammation is an essential component of host defense and immune response. However, unresolved low-grade chronic inflammation is generally viewed as a core perturbation in a range of chronic diseases [1]. Low-grade inflammation is defined as a two- to three-fold increase in circulating levels of acute phase proteins and inflammatory cytokines and other markers of immune system activity, including C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6). Low-grade systemic inflammation is associated with a number of chronic conditions, e.g. metabolic syndrome, atherosclerosis, type 2 diabetes mellitus, and nonalcoholic fatty liver disease [2,3].
Neutrophil gelatinase associated lipocalin (NGAL) is a 25 kDa glycoprotein of the lipocalin family, which was originally identified as a novel protein isolated from secondary granules of activated neutrophils. In contrast to serum creatinine, NGAL is specifically induced in the damaged nephron. Because NGAL is up-regulated shortly after damage in renal tubular cells, it is used for early detection of acute kidney injury (AKI). NGAL is a promising marker of renal epithelial injury; however, it is highly induced in a number of inflammatory diseases [4]. Recently, some investigators reported that plasma NGAL is a novel inflammatory marker for low-grade inflammatory conditions, such as Alzheimer’s disease and latelife depression [5].
Previous studies of NGAL have generally focused on its ability to predict worsening kidney function. There have been few studies that closely examined the role of NGAL as an inflammatory marker in low-grade inflammation. In the present study, we investigated whether plasma NGAL accurately reflects the severity of inflammation in low-grade inflammation, particularly based on the inflammatory markers of high-sensitivity CRP (hsCRP), TNF-α, IL-6, and corrected erythrocyte sedimentation rate (cESR).
Materials and Methods
Study population
The study included 184 patients under clinical investigation of systemic inflammation who had no renal dysfunction. Their ages ranged from 21 to 89 years (median age, 64 years), and 93 patients were male (50.5%). Age- and sex-matched healthy individuals (n=20), who had no evidence of inflammation or renal dysfunction, were enrolled as a control group. The patients were admitted to the hospital via emergency room or outpatient departments, and suffered from the following diseases: pneumonia (n=52), upper respiratory tract infection (n=44), urinary tract infection (n=36), acute hepatitis (n=25), acute pyelonephritis (n=15), acute cholecystitis (n=7), and acute pancreatitis (n=5). Clinical and demographic data were collected from medical records. Patients with recent surgery (n=3), prescription medication use (n=17), and cardiovascular disease (n=8) were excluded from the study because these conditions may influence plasma NGAL levels. Several parameters including NGAL, TNF-α, IL-6, hsCRP, cESR, serum creatinine (sCr), and estimated glomerular filtration rate (eGFR) were measured. This study was approved by the institutional review board of our university hospital.
Sample collection and laboratory investigation
Blood samples were collected from patients at admission, immediately centrifuged, and stored in aliquots at -80°C until assay. All specimens were obtained before treatment. Plasma NGAL concentrations were measured by a fluorescence immunoassay using the Triage NGAL assay (Alere, Inc., San Diego, CA, USA), which analyzes plasma NGAL with a measurable range from 15 ng/mL to 1300 ng/mL. The intraassay CVs (n=20) for three samples (mean NGAL, 68-547 ng/mL) were 4.2-6.5%; the inter-assay CVs calculated from duplicate results in 10 subsequent assays were 4.6-7.1%. A medical decision point was regarded as 150 ng/mL [6].
Serum hsCRP level was determined by the particle-enhanced immunonephelometry assay (Dade Behring, Inc, Deerfield, IL, USA). ESR was measured by the Westergren sedimentation technique using StaRRsed Auto-Compact (Mechatronics Manufacturing BV, Zwaag, The Netherlands). The cESR was calculated, based on a normal hematocrit of 45%, from the following formula: cESR (mm/h)=(subject’s hematocrit /45) × ESR (mm/h). An elevated level of hsCRP and cESR was defined as >0.3 mg/dL and >15 mm/h, which was based on the cutoff value of the 95% confidence interval for hsCRP and cESR of healthy individuals. The sCr and hsCRP levels were analyzed with a chemical analyzer (Hitachi 7600; Hitachi, Tokyo, Japan). Plasma concentrations of IL-6 and TNF-α were measured using commercially available enzyme-linked immunosorbent assay kits (R & D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. An increase in the levels of IL-6 and TNF-α was defined as >4.8 pg/mL and >10.5 pg/mL, based on the median values of the corresponding parameters in patient populations included in this study.
The grade of inflammation was determined by an inflammation index using hsCRP and cESR [7]. Scores (0.5, 1, 1.5, and 2) were assigned to each patient by the hsCRP and cESR levels: patients with hsCRP<0.3 mg/dL (score 0.5), 0.3-5.0 mg/dL (score 1.0), 5.1-10.0 mg/dL (score 1.5), and >10.0 mg/dL (score 2.0); patients with cESR<15.0 mm/h (score 0.5), 15.0-30.0 mm/h (score 1.0), 30.1-60.0 mm/h (score 1.5), and >60.0 mm/h (score 2.0). The inflammation index was obtained from the sum of the individual scores.
The subject populations were classified into two groups: lowgrade (inflammation index, >1.0 to <2.5; n=88) and high-grade (inflammation index, ≥ 2.5 to 4.0; n=96). Low-grade inflammation was determined based on the 95% confidence interval of the inflammation index that was obtained from the patients with the low-grade inflammatory diseases, including cardiovascular disease, type 2 diabetes mellitus, and atherosclerosis. The eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) formula: eGFR=186 × (sCr (mg/dL))-1.154 × (age (years))-0.203. An impaired renal function was defined as an eGFR level<60 ml/min/1.73 m2 [8].
Statistical analysis
Data were expressed as mean ± standard deviation (SD) or median value (interquartile range). Normality was confirmed by the Shapiro-Wilk test. Categorical variables were presented as frequencies and percentages. A Mann-Whitney U test and a Student’s t-test were used to analyze the data between the two groups. A multivariate regression analysis between NGAL and inflammatory parameters was conducted to allow adjusting for potential confounders, such as age, sex, body mass index (BMI), systolic blood pressure, and eGFR. A receiver operating characteristics (ROC) curve was analyzed to assess the diagnostic ability of NGAL and hsCRP to identify an elevation of IL-6 (>4.8 pg/mL) and TNF-α (>10.5 pg/mL) in patients with inflammation. Data were analyzed using SPSS software (IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY, USA). A value of P<0.05 was considered statistically significant.
Results
NGAL, TNF-α, and IL-6 in study population
The baseline characteristics of the study population are summarized in Table 1. Of the 184 patients, 114 (61.9%) had an elevated NGAL level, and 159 (86.4%) had an increase in hsCRP concentration (P<0.001). The concentrations of NGAL, TNF-α, and IL-6 were significantly higher in patients than in healthy controls (P<0.001). There were no significant differences in demographic variables between the groups (Table 1).
| Patients (n=184) | Healthy controls (n=20) | P value | |
|---|---|---|---|
| Demographic data | |||
| Age (years) | 64 (26-79) | 63 (25-81) | 0.527 |
| Sex (male; %) | 93 (50.5) | 11 (55.0) | 0.361 |
| BMI (kg/m2) | 22.4 ± 3.5 | 22.7 ± 3.2 | 0.569 |
| Systolic BP (mmHg) | 128.2 ± 27.6 | 121.3 ± 24.1 | 0.145 |
| Lipocalin | |||
| NGAL (ng/mL) | 192.0 (97.0-514.8) | 68.0 (51.7-105.2) | <0.001 |
| Inflammatory markers | |||
| hsCRP (mg/dL) | 3.27 (0.42-9.15) | 0.07 (0.04-0.12) | <0.001 |
| cESR (mm/h) | 21.5 (9.7-40.9) | 4.9 (2.1-6.4) | <0.001 |
| Cytokines | |||
| TNF-α (pg/mL) | 10.5 (4.5-31.7) | 2.4 (2.3-5.2) | <0.001 |
| IL-6 (pg/mL) | 4.8 (2.1-12.4) | 1.1 (0.9-2.6) | <0.001 |
| Renal indices | |||
| eGFR (mL/min/1.73 m2) | 72.4 (65.1-87.5) | 85.1 (72.5-96.8) | <0.001 |
| Serum creatinine (mg/dL) | 1.03 (0.80-1.26) | 0.84 (0.72-1.16) | <0.001 |
| Prevalence (n, %) | |||
| NGAL>150 ng/mL | 114 (61.9)a | 1 (5.0) | <0.001 |
| hsCRP>0.3 mg/dL | 159 (86.4) | 0 (0.0) | <0.001 |
| cESR>15 mm/h | 133 (72.3) | 0 (0.0) | <0.001 |
aSignificant (P<0.001) versus hsCRP>0.3 mg/dL.
BMI: Body Mass Index; BP: Blood Pressure; NGAL: Neutrophil Gelatinase-Associated Lipocalin; hsCRP: High Sensitivity C-Reactive Protein; cESR: Corrected Erythrocyte Sedimentation Rate; eGFR: Estimated Glomerular Filtration Rate; TNF-α: Tumor Necrosis Factor-α; IL-6: Interleukin-6.
Table 1: Baseline characteristics of study population.
NGAL and hsCRP in different grades of inflammation
The median levels of NGAL, TNF-α, IL-6, and hsCRP in highgrade inflammation were significantly elevated compared to those in low-grade inflammation. The positive rate of NGAL in low-grade inflammation was 27.3%, prominently lower than that of hsCRP (73.8%, P<0.001). However, no significant difference was observed between the positive rate of NGAL and hsCRP in high-grade inflammation (93.8% versus 97.9%, P=0.154). Median hsCRP level in high-grade inflammation was 8.8-times higher than that in low-grade inflammation, in contrast to plasma NGAL levels (3.4-times) between the corresponding groups (Table 2).
| Grades of inflammation | |||
|---|---|---|---|
| Low (inflammation index; >1.0 to <2.5; n=88) |
High (inflammation index; ≥ 2.5 to 4.0; n=96) | P value | |
| Inflammation index | 1.66 ± 0.24 | 3.04 ± 0.53 | <0.001 |
| NGAL (ng/mL) | 103.5 (69.5-159.0) | 351.9 (198.5-852.4) | <0.001 |
| Cytokines | |||
| TNF-α (pg/mL) | 5.7 (3.9-7.8) | 17.8 (5.9-40.1) | <0.001 |
| IL-6 (pg/mL) | 3.5 (1.6-3.4) | 9.2 (2.4-29.5) | <0.001 |
| Inflammatory markers | |||
| hsCRP (mg/dL) | 1.16 (0.30-3.27) | 10.25 (2.41-22.67) | <0.001 |
| cESR (mm/h) | 14.7 (7.6-21.0) | 43.1 (19.3-61.8) | <0.001 |
| Prevalence (n, %) | |||
| NGAL>150 ng/mL | 24 (27.3)a | 90 (93.8) | <0.001 |
| hsCRP>0.3 mg/dL | 65 (73.8) | 94 (97.9) | <0.001 |
| cESR>15 mm/h | 41 (46.6) | 92 (95.8) | <0.001 |
aSignificant (P<0.001) versus hsCRP>0.3 mg/dL.
NGAL: Neutrophil Gelatinase-Associated Lipocalin; hsCRP: High Sensitivity C-Reactive Protein; cESR: Corrected Erythrocyte Sedimentation Rate; eGFR: Estimated Glomerular Filtration Rate; TNF-α: Tumor Necrosis Factor-α; IL-6: Interleukin-6.
Table 2: NGAL, cytokines, and inflammatory markers by grades of inflammation.
Regression analysis
In high-grade inflammation, plasma NGAL levels were significantly correlated with IL-6 (r=0.413, P<0.001), TNF-α (r=0.325, P<0.001), and hsCRP (r=0.432, P<0.001) after adjusting for potential confounders, such as age, sex, BMI, systolic blood pressure, and eGFR. However, in low-grade inflammation, no significant associations were noted between plasma NGAL concentrations and the levels of cytokines and inflammatory markers (Table 3).
| Univariate | Multivariatea | |||
|---|---|---|---|---|
| Low grade | High grade | Low grade | High grade | |
| hsCRP (mg/dL) | 0.152 (0.103) |
0.495 (<0.001) |
0.086 (0.520) |
0.432 (<0.001) |
| cESR (mm/h) | 0.126 (0.195) |
0.467 (<0.001) |
0.074 (0.613) |
0.391 (<0.001) |
| TNF-α (pg/mL) | 0.124 (0.208) |
0.392 (<0.001) |
0.115 (0.264) |
0.325 (<0.001) |
| IL-6 (pg/mL) | 0.138 (0.141) |
0.481 (<0.001) |
0.117 (0.245) |
0.413 (<0.001) |
aAdjusted for age, sex, BMI, systolic BP, and eGFR.
hsCRP: High Sensitivity C-Reactive Protein; cESR: Corrected Erythrocyte Sedimentation Rate; eGFR: Estimated Glomerular Filtration Rate; TNF-α: Tumor Necrosis Factor-α; IL-6: Interleukin-6.
Table 3: Multivariate regression analysis of plasma NGAL levels and physiologic parameters by severity of inflammation.
ROC curve analysis
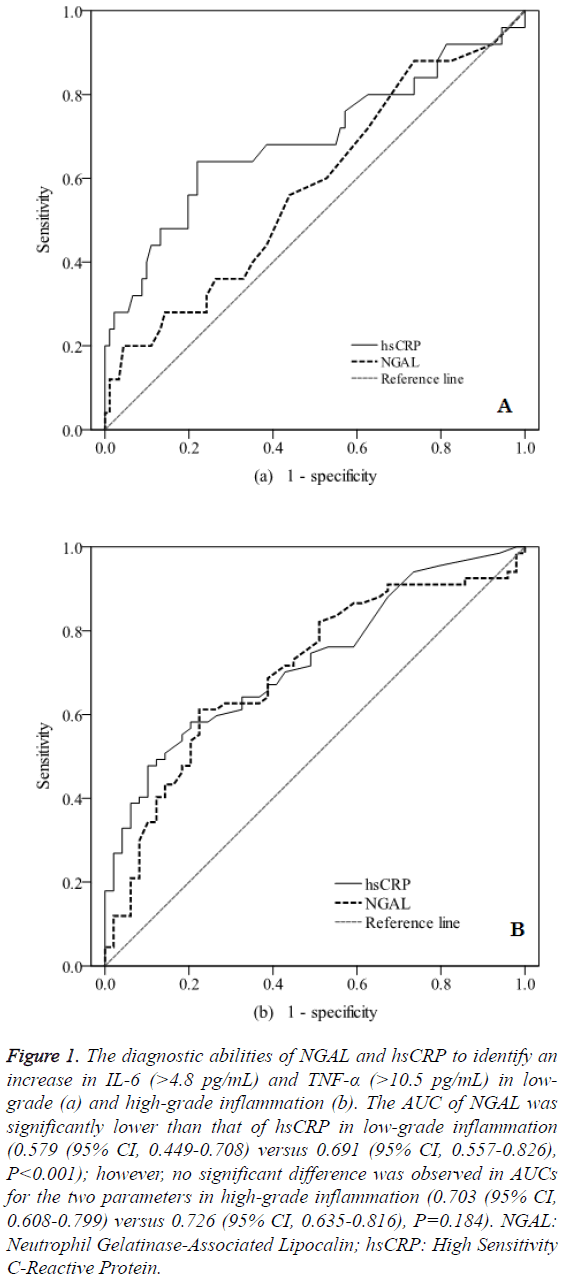
The diagnostic values of NGAL and hsCRP to identify an increase in IL-6 (>4.8 pg/mL) and TNF-α (>10.5 pg/mL) in patients with inflammation were investigated. The area under the curve (AUC) of NGAL was significantly lower than that of hsCRP in low-grade inflammation (0.579 (95% CI, 0.449-0.708) versus 0.691 (95% CI, 0.557-0.826), P<0.001). However, there were no significant differences between the AUCs of NGAL and hsCRP in high-grade inflammation (0.703 (95% CI, 0.608-0.799) versus 0.726 (95% CI, 0.635-0.816), P=0.184) (Figure 1).
Figure 1: The diagnostic abilities of NGAL and hsCRP to identify an increase in IL-6 (>4.8 pg/mL) and TNF-α (>10.5 pg/mL) in lowgrade (a) and high-grade inflammation (b). The AUC of NGAL was significantly lower than that of hsCRP in low-grade inflammation (0.579 (95% CI, 0.449-0.708) versus 0.691 (95% CI, 0.557-0.826), P<0.001); however, no significant difference was observed in AUCs for the two parameters in high-grade inflammation (0.703 (95% CI, 0.608-0.799) versus 0.726 (95% CI, 0.635-0.816), P=0.184). NGAL: Neutrophil Gelatinase-Associated Lipocalin; hsCRP: High Sensitivity C-Reactive Protein.
Discussion
In this study, the relationship between plasma NGAL levels and inflammatory parameters was investigated in patients with different grade of inflammation. The grade of inflammation was determined by a new scoring system of an inflammation index using hsCRP and cESR. A significant association was observed between NGAL and the levels of TNF-α, IL-6, and hsCRP in high-grade inflammation, but not in low-grade inflammation. The diagnostic ability of NGAL to identify an increase in IL-6 and TNF-α did not outperform that of hsCRP in low-grade inflammation. The results suggest that plasma NGAL does not accurately reflect the severity of inflammation in low-grade inflammation. NGAL is an early predictor for acute kidney damage; however, a wide heterogeneity in its predictive value is reported [6,9]. Several researchers observed that plasma NGAL can increase in the absence of tubular damage [10]. Moreover, it is unclear whether NGAL is a more crucial as an indicator of AKI than as a marker for inflammation. A group of investigators recently reported that measurement of plasma NGAL is helpful for assessing patients with low-grade inflammatory diseases [11].
Low-grade inflammation is a term for conditions in which cytokine-induced acute-phase response is ongoing. However, the scope of the condition is extremely broad and ambiguous. Clinically, low-grade systemic inflammation, mainly characterized by an elevated CRP level, is commonly associated with developing cardiovascular disease [12,13]. Duncan et al. [14] designed a classification method to indicate low-grade inflammation using several parameters including leukocytes, CRP, fibrinogen, and IL-6. In our study, we defined low-grade inflammation as the score (>1.0 to <2.5) of an inflammation index, based on the levels of hsCRP and cESR. In the current study, 47.8% of patient populations had lowgrade inflammation, 2.4- to 3.2-times higher levels in TNF-α and IL-6 compared to healthy individuals.
Catalán et al. [15] reported that low-grade inflammation leads to an increased gene expression of NGAL in patients with obesity. In our study, there were no significant associations between plasma NGAL concentrations and the levels of IL-6, TNF-α, hsCRP, and cESR in patient with low-grade inflammation. These findings may reflect the differences in study populations, severity of disease, and detection methods of NGAL, particularly between gene expression and circulating plasma level of NGAL. Additionally, in the present study, the positive rate of NGAL in patients with low-grade inflammation was significantly lower than that of hsCRP. These results imply that plasma NGAL does not correctly represent the extent of inflammation in patients with low-grade inflammation.
Proinflammatory cytokines, such as TNF-α and IL-6, are crucial in inflammatory reaction. TNF-α stimulates the secretion of IL-6, which contributes to ischemia-reperfusion injury as a multifunctional cytokine [16]. In the present study, the ability of NGAL to detect an increase in IL-6 and TNF-α was investigated using an ROC curve analysis. The hsCRP exhibited a better diagnostic performance than NGAL in
patients with low-grade inflammation, but the AUC of hsCRP was similar to that of NGAL in high-grade inflammation. These observations indicate that plasma NGAL is not superior to hsCRP for assessing low-grade inflammation, although the diagnostic efficacy of NGAL is comparable to that of hsCRP in high-grade inflammation. A measure of plasma NGAL does not seem to offer any clinical advantage over hsCRP, at least for screening low-grade inflammation. Inflammation is a risk factor for decreased renal function [17]. Systemic inflammation is frequently accompanied by kidney injury owing to its pathogenesis of hypoperfusion, apoptosis, microvascular thrombosis, and infiltration of immune cells [18]. In our study, to minimize the influence of kidney function on plasma NGAL levels, only subjects without renal impairment were enrolled. Our data obtained in patients with high-grade inflammation, who had well-preserved renal function, suggest that NGAL is a potential indicator for inflammation, but NGAL may be confined to the advanced degree of inflammation.
There are several limitations in this study. We did not measure plasma NGAL level in serial samples to assess the changes in NGAL in relation to the progression of disease. There may be unmeasured confounders for which we could not adjust during statistical analysis. Additionally, we emphasize that plasma NGAL level might be influenced by possibly missing information on renal or other organ injuries. Despite these limitations, our data may provide additional information for the diagnostic competence of NGAL in different grades of inflammation. In conclusions, this study demonstrates that plasma NGAL has sensitivity comparable to hsCRP in highgrade inflammation, but does not surpass the diagnostic accuracy of hsCRP in low-grade inflammation. The clinical relevance of NGAL as an inflammatory marker appears to be limited according to the intensity of inflammation. Thus plasma NGAL should be used with care as a biomarker to screen for patients with low-grade inflammation.
Acknowledgment
This study was supported by a research grant from Inha University.
References
- Medzhitov R. Origin and physiological roles of inflammation. Nature 2008; 454: 428-435.
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352: 1685-1695.
- Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol 2005; 98: 1154-1162.
- Giasson J, Li GH, Chen Y. Neutrophil gelatinase-associated lipocalin (NGAL) as a new biomarker for non-acute kidney injury (AKI) diseases. Inflamm Allergy Drug Targets 2011; 10: 272-282.
- Naude PJ, Eisel UL, Comijs HC. Neutrophil gelatinase-associated lipocalin: A novel inflammatory marker associated with late-life depression. J Psychosom Res 2013; 75: 444-450.
- Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009; 54: 1012-1024.
- Choi JW, Fujii T, Fujii N. Corrected neutrophil gelatinase-associated lipocalin (NGAL) level adjusted by the scoring system of an inflammation index for screening renal dysfunction in patients with systemic inflammation. Ann Clin Lab Sci 2015; 45: 248-255.
- Tsuchikura S, Shoji T, Shimomura N, Kakiya R, Emoto M, Koyama H, Ishimura E, Inaba M, Nishizawa Y. Serum C-reactive protein and thioredoxin levels in subjects with mildly reduced glomerular filtration rate. BMC Nephrol 2010; 11: 7-14.
- Clerico A, Galli C, Fortunato A, Ronco C. Neutrophil gelatinase-associated lipocalin (NGAL) as biomarker of acute kidney injury: a review of the laboratory characteristics and clinical evidences. Clin Chem Lab Med 2012; 50: 1505-1517.
- Aydoğdu M, Gürsel G, Sancak B, Yeni S, Sarı G, Taşyürek S, Türk M, Yüksel S, Senes M, Ozis TN. The use of plasma and urine neutrophil gelatinase associated lipocalin (NGAL) and cystatin C in early diagnosis of septic acute kidney injury in critically ill patients. Dis Markers 2013; 34: 237-246.
- Choi J, Lee HW, Suk K. Increased plasma levels of lipocalin2 in mild cognitive impairment. J Neurol Sci 2011; 305: 28-33.
- Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, Teeling JL, Blaak EE, Fenech M, Vauzour D, McArdle HJ, Kremer BH, Sterkman L, Vafeiadou K, Benedetti MM, Williams CM, Calder PC. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr 2015; 31: 1-14.
- Arts MH, Collard RM, Comijs HC, Naudé PJ, Risselada R, Naarding P, Oude Voshaar RC. Relationship between physical frailty and low-grade inflammation in late-life depression. J Am Geriatr Soc 2015; 63: 1652-1657.
- Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A. Low-grade systemic inflammation and the development of type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes 2003; 52: 1799-1805.
- Catalán V, Gómez-Ambrosi J, Rodríguez A, Ramírez B, Rotellar F, Valentí V, Silva C, Gil MJ, Salvador J, Frühbeck G. Six-transmembrane epithelial antigen of prostate 4 and neutrophil gelatinase-associated lipocalin expression in visceral adipose tissue is related to iron status and inflammation in human obesity. Eur J Nutr 2013; 52: 1587-1595.
- Pedersen M, Bruunsgaard H, Weis N, Hendel HW, Andreassen BU, Eldrup E, Dela F, Pedersen BK. Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech Ageing Dev 2003; 124: 495-502.
- Finlay S, Bray B, Lewington AJ, Hunter-Rowe CT, Banerjee A, Atkinson JM, Jones MC. Identification of risk factors associated with acute kidney injury in patients admitted to acute medical units. Clin Med 2013; 13: 233-238.
- Lerolle N, Nochy D, Guérot E, Bruneval P, Fagon JY, Diehl JL, Hill G. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intensive Care Med 2010; 36: 471-478.