- Biomedical Research (2016) Volume 27, Issue 3
Diagnosis of fatal chest pain with copeptin levels.
Ali Duman*, Seda Ozkan, Polat Durukan, Levent Avsaroglu, Sebahattin Muhtaroglu, Afsin Ipekci, Murat KoyuncuDepartment of Emergency Medicine, Adnan Menderes University, Turkey
Accepted date: March 08, 2016
Abstract
Chest pain is a clinical problem which often results in emergency department visits and is characterized by difficult differential diagnosis. Although many investigations can be used in the differential diagnosis of chest pain symptoms, it is forcing emergency physicians. Copeptin (CPP), is a 39-amino acid glycosylated peptide and a potential new serum inflammation marker. In this study, we aimed to investigate use of copeptin in differential diagnosis of fatal chest pain. This study included 150 patients presenting with chest pain at the emergency department over 1 year. The causes of life-threatening chest pain included aortic dissection, pulmonary thromboembolism, and acute myocardial infarction (MI); there were 90 patients in this category, identified as Group 1. Meanwhile, there were 60 patients with non-life-threatening, non-specific causes of chest pain, who were categorized in Group 2. As a Control Group, 30 healthy individuals with normal physical examination results were included. In terms of blood copeptin levels, the result was 0.48 ± 0.27 ng/ml for the Control Group, 1.03 ± 0.56 ng/ml for Group 1, and 0.50 ± 0.32 ng/ml for Group 2. There was a statistically significant difference between Group 1 and Group 2 (p<0.05), while no significant difference was found between Group 2 and the Control Group (p>0.05). Serum copeptin value should be used in differential diagnosis of fatal chest pain.
Keywords
Fatal chest pain, Copeptin, Inflammatory marker.
Introduction
Chest pain is a problem that is often encountered in emergency departments. Complaint of chest pain complaint comprises 5% of emergency department admissions [1]. Although numerous studies are used in differential diagnosis, chest pain is quite a compelling complaint for emergency department physicians. Among the most important difficulties encountered during the assessment of the patients with chest pain in the emergency service are that there many illnesses cause this symptom and that the diagnosis may be difficult to determine [2].
In differential diagnosis of chest pain, life-threatening states such as aortic dissection, pulmonary embolism, and acute coronary syndrome must be considered and detection during the emergency treatment and intervention planning should be the first step. However, when it comes to chest pain, there are many causes that have low mortality in addition to the more serious causes that must be recognized quickly and treated immediately. In this group, chest pain may be of skeletal system, gastrointestinal system, or psychogenic origin. These diagnoses are not usually examined in emergency departments, and after fatal situations are excluded, patients are discharged with a diagnosis of nonspecific chest pain [1]. Most patients with chest pain receive such a diagnosis [3,4].
Copeptin is a biomarker that is cosynthesized with arginine vasopressin (AVP) and secreted from the posterior pituitary gland. The copeptin AVP precursor represents the diversion of the stable. Copeptin is stable in plasma and serum. Thus, the level of AVP is easily measurable using a simple immunometric method. Recently, various studies have been performed to evaluate copeptin levels, a new marker in different diseases such as acute coronary syndrome, chronic obstructive pulmonary disorder (COPD), sepsis, and shock. In the literature, no broad study about the causes of chest pain is available [5-7].
In this study, we aimed to investigate whether the copeptin value of blood used should be used for the differential diagnosis of chest pain.
Materials and Methods
This clinical study was carried out at the emergency service and supported by the Erciyes University Research Fund (TSU-10-2977) after getting ethics committee approval.
Patients admitted to the emergency department with a complaint of chest pain over 1 year were considered. Participants were included in the study after consent was given by the patient and/or the patient’s relatives.
The exclusion criteria were patients under the age of 18, renal failure, congestive heart failure, malignity, liver failure, and pregnancy or probable pregnancy.
One hundred and fifty patients were included in the study. The patients were split into two groups. Ninety patients with a diagnosis of life-threatening causes of chest pain, such as acute MI, pulmonary thromboembolism, and aortic dissection, were included in Group 1. Sixty patients with a diagnosis of nonspecific or nonfatal chest pain were included in Group 2. Thirty healthy persons were included as the Control Group.
Patients presenting with chest pain were examined after providing respiratory, circulatory, and airway security. Vascular access, monitoring, and oxygen were provided for the patients. The patients’ electrocardiograms (ECGs) and lung images were evaluated. Blood samples were taken from the patients to investigate creatine kinase (CK), creatine kinase MB (CKMB), troponin, D-dimer, and copeptin levels.
D-Dimer, CK, CK-MB, troponin, and copeptin were studied immediately at the Gulser–M.D. Mustafa Gundogdu Center Laboratory. A 5 ml venous blood sample was taken in an EDTA tube to evaluate copeptin. After the EDTA tube was, the blood was transferred to a centrifuge tube and centrifuged at 3,500 cycles for 10 minutes and the serum was separated. Samples were kept at -70°C before testing. Copeptin samples were dissolved at room temperature on the day of examination. Copeptin levels were measured using the sandwich enzymelinked immunosorbent assay (ELISA) method at Gulser the Gulser–M.D. Mustafa Gundogdu Center Laboratory using an EK-065-32 Human Copeptin ELISA kit from Alpina.
Statistical analysis
The SPSS 15.0 and SigmaStat 3.5 statistical package programs were used to evaluate the data. Median, percentage, standard deviation (SD), mean, maximum and minimum values, and 25–75 percentile values were used as descriptive statistics. Kruskal–Wallis analysis was used to analyze the non-normallydistributed copeptin variables for the three groups. The Chisquare exact method was used for the comparison of the groups according to qualitative variables. A value of p<0.05 was accepted as statistically significant.
Results
In Group 1, out of the 90 patients, 68 (75.55%) were diagnosed with acute MI, 15 (16.67%) with pulmonary thromboembolism, and 7 (7.78%) with aortic dissection. In Group 2, of 60 patients, 29 (48.33%) were diagnosed with myalgia, 12 (20%) with peptic ulcer and gastroesophageal reflux, 4 (6.67%) with pneumonia, and 15 (25%) with anxiety.
The mean age of patients was 61.40 ± 13.31 years in Group 1 and 51.41 ± 15.80 years in Group 2. One hundred and two (68%) patients were male and 48 (32%) were female. In terms of group composition, 68 (45.33%) patients were male and 22 (14.67%) patients were female in Group 1, while 34 (22.67%) patients were male and 26 (17.33%) were female in Group 2.
There was a statistically significant age difference between the groups (p<0.001). However, there was no statistically significant gender difference between the groups (p=0.20; Table 1).
| Male | Female | TOTAL | p | ||||
|---|---|---|---|---|---|---|---|
| GROUPS | n | % | n | % | n | % | P=0,200 |
| Group 1 | 68 | Â 45,33 | 22 | 14,67 | 90 | Â 60 | |
| Group 2 | 34 | 22,67 | 26 | Â 17,33 | 60 | 40 | |
| TOTAL | 102 | 68 | 48 | Â 32 | 150 | 100 | |
p<0.001 was accepted stastically significant.
Table 1. Gender Distribution of Groups.
When the mean ± SD of the patients’ biochemical parameters were reviewed, a statically significant difference was determined between Groups 1 and 2 in relation to parameters such as troponin-T, CK, D-dimer, lactate dehydrogenase (LDH), aspartate aminotransferase (AST), CK-MB (p<0.001; Table 2).
| Group 1 Mean ± SD | Group 2 Mean ± SD | P | |
|---|---|---|---|
| CK (µ/L) | 653,20 ± 1044, 43 | 107,15 ± 134,90 | p<0,001 |
| CK-MB (µ/L) | 91,13 ± 124,49 | 19,23 ± 11,49 | p<0,001 |
| Troponine-T (ng/L) | 1,65 ± 3,70 | 0,01 ± 0,01 | p<0,001 |
| D-Dimer (µg/L) | 2457,22 ± 4637,59 | 578,33 ± 817,67 | p<0,001 |
| LDH (µ/L) | 418,36 ± 337,02 | 262,60 ± 83,23 | p<0,001 |
| AST (µ/L) | 94,27 ± 274,30 | 34,68 ± 78,85 | p<0,001 |
p<0.001 was accepted stastically significant.
Table 2. Mean Values of Biochemical Parameters of Groups.
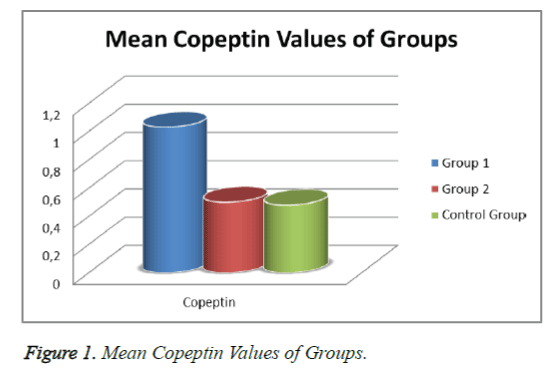
When the patient groups and controls were evaluated in terms of mean copeptin levels ± SD, the levels were 1.03 ± 0.56 ng/ml in Group 1, 0.50 ± 0.32 ng/ml in Group 2, and 0.48 ± 0.27 ng/ml in the Control Group. While statistically significant differences were determined between Groups 1 and 2 and between Group 1 and the Control Group (p<0.05), there was no statistically significant difference between Group 2 and the Control Group in terms of copeptin levels (p>0.05; Figure 1).
Discussion
Chest pain–related admissions constitute 5% of emergency department admissions [1,2]. Most patients admitted to emergency departments with chest pain are discharged with a diagnosis of nonspecific chest pain [8]. Although patients discharged with such a diagnosis have a low mortality rate, studies have shown that these patients have frequent hospital admissions and recurrent (repetitive) diagnostic examinations [4,9]. In previous research, 40–60% of patients who presented at the hospital with chest pain were found to have nonspecific chest pain [3,4]. In this study, 60% of the patients admitted to the emergency department with chest pain were diagnosed with life-threatening chest pain and 40% of patients were diagnosed with non-life-threatening chest pain.
Pulmonary embolism, which is a cause of life-threatening chest pain in adults, can be seen almost in all age groups, but 63% of the patients are over 60 years old. Over this age, incidence of this disease is more frequent in males and increases with age in both sexes [10]. The male-to-female ratio of aortic dissection is 3:1 and its peak age ranges from the sixth decade to the seventh decade [11,12]. Acute coronary syndrome exhibits some differences depending on age and sex. While the mean age of a first heart attack in males is 65.87 years, it is 70.4 years in females [13].
Reichlin et al. reported that the mean age of these patients was 62 ± 17 years. Sixty-six percent of the patients were male and 44% were female [14]. Khan et al. reported that the mean age of these patients was 66 years of age. In their study, 73% of the patients were male and 27% of the patients were female [5]. Chai et al. reported that the mean age of these patients was 61±11 years old. Thirteen patients were male and 8 were female [15]. In our study, life-threatening chest pain was seen more in advanced age and in males. This is compatible with the data in the literature.
Certain blood parameters are used in the diagnosis of potentially fatal chest pain. Although increased cardiac troponins, CK, and CK-MB are routinely used and are helpful in the diagnosis of acute coronary syndrome, they may not rise in early hours of acute MI. Therefore, their benefits are limited in terms of early diagnosis [16]. The detection or exclusion of myocardial damage can be done via serial measurement using traditional markers such as CK and CK-MB. However, this is a time consuming protocol [17]. Laboratory studies such as sedimentation, leukocyst count, LDH, AST, and bilirubin count may support the diagnosis of pulmonary thromboembolism but are not specific to it. D-dimer is a specific degradation product of fibrin. D-Dimer levels may rise as much as eightfold in cases pulmonary thromboembolism. The sensitivity of D-dimer over 500 ng/ml in the diagnosis of pulmonary thromboembolism is 97–100%. However, D-dimer tests have shown a low sensitivity in 35–45% of cases [18]. The negative predictive value of D-dimer in patients with a clinically high probability of pulmonary thromboembolism is too low to safely exclude it [19]. Studies have shown that D-dimer especially increases in aortic dissection [20]. In this study, CK, CK-MB, LDH, troponin, D-dimer, and AST were analyzed in cases of life-threatening chest pain. When the biochemical parameters between the groups were analyzed, it was found that there was statistically significant difference between the groups (p<0.001). In patients with potentially fatal diseases, LDH, AST, D-dimer, troponin-T, CK, and CK-MB levels were considerably higher than in patients with non-life-threatening diseases. These findings were compatible with the data in the literature.
AVP is released due to neurohypophysis. It is important in renal osmoregulation and cardiovascular homeostasis the in renal fluid balance [21]. The AVP level increases in congestive heart failure and acute MI. Some difficulties may arise while measuring and detecting AVP levels [22]. In contrast, it is easier and faster to measure competing levels. Thus, determining copeptin levels is an alternative to direct measurement of AVP [23].
Recently, various studies have been performed to evaluate copeptin levels, a new marker in different diseases such as acute coronary syndrome, COPD, sepsis, and shock. In the literature, no broad study has been published about the causes of chest pain. Khan et al. reported that copeptin levels were high in patients who developed acute MI and died [5]. Chai et al. showed that plasma copeptin levels were considerably higher in the coronary artery disease group compared to the control group. Copeptin levels were significantly higher after the first day of Percutaneous transluminal coronary angioplasty and stent treatment. After the third and fifth days of the treatment, copeptin was still high in the treated group compared to the control group, although these levels did decrease [15]. Reichlin et al. showed that plasma copeptin levels combined with troponin-T were more specific and sensitive than troponin-T alone in the exclusion of acute MI diagnosis [14].
This study aimed to determine whether copeptin might be used in differential diagnosis of life-threatening and non-lifethreatening chest pain. It was found that plasma copeptin levels declined in patients with potentially fatal chest pain. Moreover, it was observed that the high level of plasma copeptin was statistically significant in the life-threatening chest pain group (Group 1) compared to both controls and the non-lifethreatening chest pain group (Group 2).
In conclusion, plasma copeptin levels might be used in addition to other parameters in the differential diagnosis of potentially fatal chest pain and non-life-threatening chest pain.
References
- Green GB, Hill PM. Cardiovascular disease: Approach to chest pain and possible myocardial ischemia. In; Emergency Medicine: A Comprehensive Study Guide, 5th ed. Tintinalli JE, Kelen GD, Stapczynski JS (eds). North Carolina: McGraw-Hill 1999; 341-351.
- O’Rourke RA, Shaver JA, Silverman ME. Hikaye, Fizik Muayene ve Oskültasyon. In; Hurst’s The Heart, 10. baskı,Türkçe. Fuster V, Alexander RW, O’Rourke RA (eds) (çeviri ed: Yılmaz Y, Şahinbaş E) McGraw-Hill 2002; 193-281.
- Solinas L, Raucci R, Terrazzino S, Moscariello F, Pertoldi F, Vajto S, Badano LP. Prevalence, clinical characteristics, resource utilization and outcome of patients with acute chest pain in the emergency department. A multicenter, prospective, observational study in north-eastern Italy. Ital Heart J 2003;4:318-324.
- Eslick GD, Fass R. Noncardiac chest pain: Evaluation and treatment. Gastroenterol Clin North Am 2003; 32:531-552.
- Khan SQ, Dhillon OS, O’Brien RJ, Struck J, Quinn PA, Morgenthaler NG, Squire IB, Davies JE, Bergmann A. C-terminal Provazopressin (Copeptin ) as a Novel and Prognostic Marker in Acute Myocardial Infarction. Circulation 2007;115:2103-2110.
- Westermann I, Dünser MW, Haas T, Jochberger S, Luckner G, Mayr VD, Wenzel V, Stadlbauer KH, Innerhofer P, Morgenthaler N, Hasibeder WR, Voelckel WG. Endogenous vasopressin and copeptin response in multiple trauma patients. Shock 2007; 28:644-649.
- Katan M, Müller B, Christ-Crain M. Copeptin: a new and promising diagnostic and prognostic marker. Critical Care 2008;12:117.
- Kontos MC. Evaluation of the Emergency Department chest pain patient. Cardiol Rev 2001; 9: 266-275.
- Wong WM, Lam KF, Cheng C, Hui WM, Xia HH, Lai KC, Hu WH, Huang JQ, Lam CL, Chan CK, Chan AO, Lam SK, Wong BC. Population based study of noncardiac chest pain in southern Chinese: prevalence, psychosocial factors and health care utilization. World J Gastroenterol 2004 ; 10:707-712.
- Blann AD, Lip GYH. Venous thromboembolism. BMJ 2006; 332: 215-219.
- Sutherland A, Escano J, Coon TP. D-dimer as the sole screening test for acute aortic dissection: a review of the literature. Ann Emerg Med. Oct 2008;52:339-343.
- Von Kodolitsch Y, Nienaber CA, Dieckmann C, Schwartz AG, Hofmann T, Brekenfeld C, Nicolas V, Berger J, Meinertz T. Chest radiography for the diagnosis of acute aortic syndrome. Am J Med. Jan 15 2004;116:73-77.
- Heart and Stroke Statistics For Women. American Heart Association Heart Disease and Stroke Statistics-2006 Update.
- Reichlin T, Hochholzer W, Stelzig Z, Laule K, Freidank H, Morgenthaler NG, Bergmann A, Potocki M, Noveanu M, Breidthardt T, Christ A, Boldanova T, Merki R, Schaub N, Bingisser R, Christ M, Mueller C. Incremental Value of Copeptin for Rapid Rule Out of Acute Myocardial Infarction. Journal of the American College of Cardiology 2009; 54: 60-68.
- Chai SB, Hui YM, Li XM, Xiao Y, Tang CS. Plasma levels of copeptin in patients with coronary heart disease. Heart Vessels 2009;24: 79–83.
- Alhadi HA, Fox KAA. Do we need additional markers of myocyter necrosis: the potantial value of heart fatty acid binding protein. QJ Med 2004; 97: 187-198.
- Storrow AB, Gibler WB. Chest Pain Centers: diagnosis of acute coronary syndromes. Ann Emerg Med 2000; 35: 449-461.
- Zimmerman J, Fromm, Mayer D, Ann Boudreaux, Chuan-Chuan CW, Smalling R,Davis B, Habib G, Roberts R.Diagnostic marker cooperative study for the diagnosis of minfarction. Circulation 1999;99:1671-1677.
- Perrier A, Desmarais S, Miron MJ, de Moerloose P, Lepage R,Slosman D, Didier D, Unger PF, Patenaude JV, Bounameaux H. Noninvasive diagnosis of venous thromboembolismin outpatients. Lancet 1999;353: 190-195.
- Suzuki T, Distante A, Zizza A, Trimarchi S, Villani M, Salerno Uriarte JA, De Luca Tupputi Schinosa L, Renzulli A, Sabino F, Nowak R, Birkhahn R, Hollander JE, Counselman F, Vijayendran R, Bossone E, Eagle K. Diagnosis of acute aortic dissection by D-dimer: the International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) experience. Circulation 2009;119:2702-2707.
- Robertson GL. Antidiüretic hormone. Normal and disordered function. Endocrinol Metab Clin North Am 2001;30:671-694.
- Lejemtel TL, Serro C. Vasopressin dysregulation: hyponatremia, fluid retention and congestive heart failure. Int J Cardiol 2007;120:1-9.
- Morgenthaler NG, Struck J, Jochberger S, Dünser MV. Copeptin: clinical use of a new biomarker. Trends Endocrinol Metab 2008; 19: 43-49.