Research Article - Current Pediatric Research (2022) Volume 26, Issue 9
Diagnosing ventilator-associated pneumonia in pediatric patients with limited facilities: a retrospective cohort study
Irene Yuniar*, Immanuela Hartono, Sita Febriani
Department of Pediatrics, College of Medicine, Universitas Indonesia, Dr. Cipto Mangunkusumo General Hospital, Central Jakarta, Indonesia
- *Corresponding Author:
- Irene Yuniar
Department of Pediatrics
College of Medicine
Universitas Indonesia
Dr. Cipto Mangunkusumo General Hospital
Central Jakarta
Indonesia
E-mail: irene.tambunan93gmail.com
Received: 02 September, 2022, Manuscript No. AAJCP-22-70972; Editor assigned: 05 September, 2022, PreQC No. AAJCP-22-70972(PQ); Reviewed: 12 September, 2022, QC No. AAJCP-22-70972; Revised: 19 September, 2022, Manuscript No. AAJCP-22-70972(R); Published: 27 September, 2022, DOI:10.35841/0971-9032.26.9.1633-1639.
Abstract
Background: Ventilator Associated Pneumonia (VAP) is the second leading cause of Healthcare Associated Infection in the PICU. However, there are no specific criteria for diagnosing VAP in pediatric patients. This study evaluates the sensitivity and specificity of CDC pediatric VAP definition to adult Ventilator Associated Event (VAE) criteria in diagnosing VAP in pediatric patients, the risk factors and common organisms causing VAP. Methods: A retrospective study evaluated patients aged 1 month to 12 years admitted to the PICU tertiary hospital Cipto Mangunkusumo from January 2019 to June 2019, who received mechanical ventilation for ≥ 2 days. Results: From 103 patients, 18 (13.85%) and 9 (8.74%) patients developed VAP according to VAE and CDC respectively. The specificity of VAE and CDC criteria reached 100% and 96.9% respectively with 45% and 22.5% in sensitivity. Logistic regression analysis found that duration of mechanical ventilator >5 days (OR 7.519; 95% CI:2.26,25; P=0.001), re-intubation within 72 hours after extubation (OR 4.057; 95% CI:1.36,12.09; P=0.009), usage of vasoactive drugs (OR 4.364; 95% CI:0.94,20.27; P=0.044) and proton pump inhibitor (OR 5.00; 95% CI:1.52,16.47; P=0.005) as the risk factors for VAP. Pseudomonas aeruginosa, klebsiella pneumonia and acinetobacter are the most common organisms found in the VAP group. Conclusion: VAE has higher sensitivity and specificity compared to CDC’s new criteria to diagnose VAP in pediatric patients. However, the new CDC criteria have high specificity and will help physicians in limited facilities diagnose VAP in immunocompetent pediatric patients without the need to conduct invasive examinations like BAL examinations.
Keywords
Healthcare associated infection, Mechanical ventilator, Pediatric critical care patients, Pediatric intensive care unit, Ventilator associated event, Ventilator associated pneumonia.
Introduction
Ventilator Associated Pneumonia (VAP) is the second leading disease that causes Healthcare Associated Infection (HAI) in Pediatric Intensive Care Unit (PICU) and Neonatal Intensive Care Unit (NICU) [1]. Centers for Disease Prevention and Control (CDC) reported that in 2011, HAI incidence rates reached 157.000 in United States' hospitals and VAP contributed 39% from all those cases [2]. In Indonesia, ICU RSUP Dr. Mohammad Hoesin Palembang, also reported that from 106 patients who used mechanical ventilators within 2014 July to 2015 June, 41 patients (38.7%) developed VAP and the mortality rate reached 63.4% [3]. This evidence supports the theory that HAI will increase patients' mortality, morbidity, and Length of Stay (LOS) in the hospital [4].
In 2010, Germany by the German interdisciplinary society for intensive care medicine (DIVI) then set VAP incidence rates as one of the indicators for assessment of patient safety quality [5]. This decision stirred controversies and arguments because there is no gold standard definition to diagnosing VAP in adults, moreover in pediatric patients [4]. There are several definitions and criteria to diagnose VAP, but none of those criteria is specific for pediatric patients. As a result, there is a probability that the reported cases of VAP are not an actual image of cases in the field because the incidence of VAP is very variable even in one hospital; depending on the criteria they used [6].
DIVI revised indicators for assessment of patient safety in 2013 by removing incidence rates of VAP and set ventilator bundles; which is a guideline to prevent or reduce VAP rates in ICU; as one of the new indicators. Later, the indicator kept changing and since 2017, ventilator bundles has been extended, and "early weaning from mechanical ventilation" and "Prevention of infection in intensive care unit" became the new indicator for assessment of patient safety [7].
On the other side, the definition of VAP still going under revision until now because physicians complained about the over diagnosis and under diagnoses VAP cases reported [1]. In 2019, CDC published a Ventilator Associated Event (VAE) algorithm for diagnosing patients with Possible Ventilator Associated Pneumonia (PVAP). However, in this algorithm, they already stated that this algorithm is made for adult patients only [8].
In the case of diagnosing VAP in pediatric patients, in the same year, CDC also released a module for diagnosing VAP and non-ventilator-associated Pneumonia (PNU) events for adult and pediatric patients which undergo some revision in early 2021 [9]. Even though this module can help to diagnose VAP in pediatric patients, still, this algorithm has the same algorithm between diagnosing VAP and PNEU with the condition of patient need to be under a mechanical ventilator for at least 2 calendar days to be called VAP instead of PNEU [2].
Furthermore, the PNEU algorithm also specified the algorithm between non-immuno compromised with immune compromised patients and also can be used to diagnose even patients with pulmonary diagnosis as an underlying disease. On the other hand, this PNEU algorithm for non-immuno compromised patients does not include laboratory testing like the VAE algorithm to diagnose VAP. This might be beneficial if the sensitivity and specificity can be as good as or even better than the VAE algorithm, because BAL or sputum examination that is necessary for the VAE algorithm is quite expensive and invasive, or this could also be the downside for this algorithm. Therefore, we collected the data of pediatric patients in the PICU of Dr. Cipto Mangunkusumo general hospital to compare the algorithm between VAE and PNEU to diagnose them with VAP.
Methods
Data sources and patient population
This was a retrospective cohort study. After receiving approval from Universitas Indonesia's institutional ethics committee with reference number: KET-815/UN2.F1/ETIK/PPM.00.02/2019, data of pediatric patients (1 month to 12 years old) between January 2019 to June 2019 who entered PICU in Dr. Cipto Mangunkusumo general hospital, Jakarta, Indonesia who received a mechanical ventilator for a minimum of 2 calendar days were collected. Samples data from patient demographics, vital signs, diagnoses, medications, treatment plans, progress notes, PELOD-2 score, time-stamped laboratory and imaging results were obtained from Electronic Health Records (EHR) and PICU internal database.
First, PICU documentation from January 2019 to June 2019 was reviewed to obtain data of pediatric patients aged 1 month to 12 years old, who received mechanical ventilators for greater or equal to 2 calendar days. Samples were classified into patients with and without pulmonary diagnosis as an underlying disease and immunocompromised or non-immunocompromised group. For demographic variables and analysis, additional data such as age, sex, primary diagnosis, PELOD-II score, and daily progress of hospitalization, radiology report, and laboratory results were taken from EHR. In addition, we were also collected data to analyze the risk factors of VAP, such as duration of mechanical ventilation, reintubation conducted within 72 hours after extubation, history of a genetic disorder or muscular disease, the use of proton pump inhibitor, vasoactive drugs, and H-2 antagonist and oral hygiene. Minimal sample size was calculated to assess VAP incidence and a comparison between VAE and PNEU algorithm to diagnose VAP and 95 samples were required. In total, 108 samples were obtained, but only 103 samples were included in the study because of a lack of data in some samples. All methods were performed in accordance with the relevant guidelines and regulations of the declaration of Helsinki.
Outcome definitions
For the diagnosis of VAP, the CDC PNEU/VAP device-associated module of VAP definition is taken. This module uses a combination of imaging and clinical criteria for identifying Pneumonia in pediatric patients who received mechanical ventilation for greater or at least 2 calendar days on the date of the event. For a group with non-immunocompromised without pulmonary diagnosis as underlying disease, children age older than 1 year old or 12 years and younger, at least one new-onset or progressive and persistent imaging abnormality was found with three sign symptoms of pneumonia and additional symptoms of desaturation or increased oxygen requirement for 1-year-old or younger patients.
In a group with non-immunocompromised patients with pulmonary diagnosis as an underlying diagnosis, the difference is in chest imaging test results which should show at least two new-onset or progressive and persistent imaging abnormalities. For the immunocompromised group, only one symptom of pneumonia is needed, but identification for certain organisms should be found in blood and/or sputum, endotracheal aspirate, BAL, or protected specimen brush. In the end, samples that did not go through sputum or BAL culture were assumed to be negative in VAP.
Statistical model
All collected data were entered into an SPSS and were inserted into both VAE and PNEU algorithms to get a two-by-two (2 × 2) table. Thereafter, the chi-square test was used to compare variables between patients with and without VAP which were resulted in specificity, sensitivity, positive predictive value, and negative predictive value of both tests. Univariate analysis was done to assess the association of various categorical risk factors with VAP with a P-value <0.1. The final results were completed with an adjusted odds ratio (OR) with a 95% confidence interval and P-value. All analyses were conducted with SPSS version 25.0.
Results
Demographics
From 108 pediatric patients admitted to Dr. Cipto Mangunkusumo hospital PICU within January 2019 to June 2019 who use mechanical ventilation for 2 or greater than 2 days, 5 patients were excluded from the study because of lack of data. From 103 patients, VAP was diagnosed in 18 (13.85%) and 9 (8.74%) patients according to VAE and CDC respectively.
Table 1 reports the sensitivity, specificity, and positive likelihood ratio (LR) of VAE criteria compared to CDC criteria in diagnosing pediatric patients with VAP. From 40 patients who showed positive culture results in BAL and sputum, 18 patients met the criteria for VAE and 9 patients for CDC. VAE still showed higher sensitivity and specificity compared to CDC criteria in diagnosing VAP in pediatric patients. However, both criteria were not sensitive enough in diagnosing VAP, but they showed satisfactory specificity approaching 100% and 96.9% with VAE and CDC criteria respectively.
| Diagnosis | Sensitivity | Specificity | p | Positive Likehood Ratio(95% CI) |
|---|---|---|---|---|
| Probable VAP (VAE) | 45 | 100 | 0.000 | 30.5 (6.55-142.1) |
| PNEU (CDC) | 22.5 | 96.9 | 0.002 | 18 (2.181-148.54) |
Table 1. Test Characteristics of the VAE and CDC VAP definition compared to positive BAL examination.
The main characteristics of patients included in the study are presented in Table 2. This table also showed that patients who developed VAP with criteria PVAP and PNEU have a longer median duration on mechanical ventilation (4 days compared to 9 days and 11.5 days), as well as prolonged the length of ICU stay (6 days compared to 13.5 and 12.5 days). Compared to all patients with a mechanical ventilator for 2 or greater than 2 days who have a 30.1% mortality rate, VAP patients who were diagnosed with VAE and CDC also have higher mortality rates (61.1% and 70% respectively).
| Patient Characteristic, n(%) | All (n=103) | Culture Positive*(n=40 ) | PVAP Positive(n=18) | PNEU Positive (n=10) |
|---|---|---|---|---|
| Sex | ||||
| Male | 57(55.3) | 21(52.5) | 9(50) | 5(50) |
| Female | 46(44.7) | 19(47.5) | 9(50) | 5(50) |
| Age | ||||
| 1 m–1 yr | 47(45.6) | 21(52.5) | 12(66.7) | 5(50) |
| 1–12 yr | 56(54.4) | 19(47.5) | 6(33.3) | 5(50) |
| Medianage, month (IQR) | 15 (5– 56) | 10.5(4–44) | 6(4-33.75) | 23.5(3.5-100) |
| Typeof case | ||||
| Medical | 62(60.2) | 32(80) | 14(77.8) | 5(50) |
| Surgical | 41(39.8) | 8(20) | 4(22.2) | 5(50) |
| Durationof MV | ||||
| 2–4 days (early onset) | 62(60.2) | 11(27.5) | 4(22.2) | 4(40) |
| ≥ 5 days(late onset) | 41(39.8) | 29(72.5) | 14(77.8) | 6(60) |
| Medianduration of MV (IQR) | 4 (2–8) | 8 (4–18.5) | 9(5.25-21.25) | 11.5(3-25) |
| PELOD-2Score | ||||
| <10 | 98 (95.1) | 37(92.5) | 17(94.4) | 9(90) |
| ≥ 10 | 5(4.9) | 3(7.5) | 1(5.6) | 1(10) |
| MedianScore PELOD-2 (IQR) | 3 (3–5) | 5 (3–7) | 5(4.48-7.5) | 7.5(5.25-9) |
| Patientdeveloped sepsis | 31(30.1) | 19(47.5) | 12(66.7) | 4(40) |
| Lengthof PICU stay, d | 6 (4–13) | 11.5(7–25) | 13.5 (8-25.25) | 12.5(4.75-25.5) |
| Mortality | ||||
| Home | 72(69.9) | 22(55) | 7(38.9) | 3(30) |
| Death before discharge | 31(30.1) | 18(45) | 11(61.1) | 7(70) |
Table 2. Demographic and clinical features of all patients, culture positive patients and ventilator-associated pneumonia who met VAE and CDC criteria.
Risk factors
Table 3 displays the risk factors for pediatric patients who developed VAP with VAE criteria. Risk factors for VAP found in group with more than 5 days duration of mechanical ventilator, reintubation within 72 hours after extubation and group with vasoactive or proton pump inhibitor. Longer duration of mechanical ventilation will increase the risk of patients developed VAP 7.519 times compared to shorter duration/early onset. (Adjusted odd ratio 7.519; 95% CI: 2.26, 25; P=0.001), reintubation within 72 hours after extubation (adjusted odd ratio 4.057; 95% CI: 1.36, 12.09; P=0.009), usage of vasoactive drugs (adjusted odd ratio 4.364; 95% CI: 0.94, 20.27; P=0.044) and proton pump inhibitor (adjusted odd ratio 5.00; 95% CI: 1.52, 16.47; P=0.005) as the risk factors for VAP.
| Characteristic | PVAP | p | OR (95%CI) | |
|---|---|---|---|---|
| NO (n=85) | YES (n=18) | |||
| Durationof mechanical ventilator | ||||
| 2-4days (early onset) | 58(54.4) | 4(5.8) | 0.001 | 7.519(2.26-25) |
| ≥ 5days (late onset) | 27 (24.3) | 14(15.5) | ||
| PELOD-2score | ||||
| <10 | 81(78.6) | 17(16.5) | 0.879 | 1.191(0.13-11.33) |
| ≥ 10 | 4(3.9) | 1 (1) | ||
| Reintubationwithin 72 hours post extubation | ||||
| No | 71(68.9) | 10(9.7) | 0.009 | 4.057(1.36-12.09) |
| Yes | 14(13.6) | 8(7.8) | ||
| Vasoactivedrugs | ||||
| No | 30(29.1) | 2(1.9) | 0.044 | 4.364(0.94-20.27) |
| Yes | 55(53.4) | 16(15.5) | ||
| ProtonPump Inhibitor | ||||
| No | 50(48.5) | 4(3.9) | 0.005 | 5.00(1.52-16.47) |
| Yes | 35(34) | 14(13.6) | ||
| H2antagonist | ||||
| No | 63(61.2) | 13(12.6) | 0.868 | 1.101(0.35-3.44) |
| Yes | 22(21.4) | 5(4.9) | ||
| Hygieneoral | ||||
| Yes | 82(79.6) | 18(17.5) | 0.419 | 0.82(0.75-0.90) |
| No | 3(2.9) | 0 (0) | ||
| Immunocompromised | ||||
| No | 57(55.3) | 11(10.7) | 0.628 | 1.295(0.45-3.70) |
| Yes | 28(27.2) | 7(6.8) | ||
| MuscleDisorder (GBS, SMA, etc.) | ||||
| No | 82(79.6) | 16(15.5) | 0.174 | 3.417(0.53-22.12) |
| Yes | 3(2.9) | 2(1.9) | ||
| GeneticSyndrome | ||||
| No | 68(66.0) | 15(14.6) | 0.745 | 0.80(0.21-3.08) |
| Yes | 17(16.5) | 3(2.9) | ||
Table 3. Univariate analysis to assess the association of various categorical risk factors with PVAP.
Microorganism
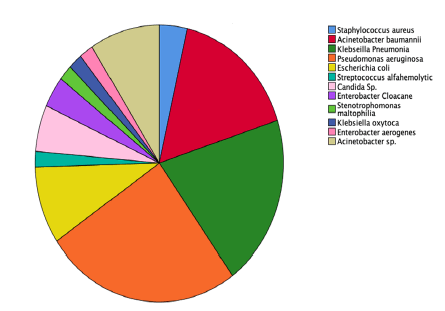
Out of 57 patients with long duration of the mechanical ventilator and symptoms of pneumonia, BAL or sputum culture examination was conducted and 40 patients showed positive results. The most number of microorganisms found were Pseudomonas aeruginosa, klebsiella Pneumonia followed by acinetobacter baumannii. BAL and sputum results showed in Table 4 and Figure 1.
| Causative organism | Frequency | Percent |
|---|---|---|
| Staphylococcusaureus | 2 | 3.6 |
| Acinetobacterbaumannii | 9 | 16.4 |
| Klebseillapneumonia | 11 | 20 |
| Pseudomonasaeruginosa | 14 | 25.5 |
| Escherichiacoli | 5 | 9.1 |
| Streptococcusalfahemolytic | 1 | 1.8 |
| Candidasp. | 3 | 5.5 |
| Enterobactercloacane | 2 | 3.6 |
| Stenotrophomonasmaltophilia | 1 | 1.8 |
| Klebseillaoxytoca | 1 | 1.8 |
| Enterobacteraerogenes | 1 | 1.8 |
| Acinetobactersp. | 5 | 9.1 |
| Total | 55 | 100 |
Table 4. Causative organisms and frequency found in positive culture patients.
Discussion
From 103 pediatric patients who were under a mechanical ventilator for 2 or greater than 2 days, 18 patients were diagnosed with VAP with VAE criteria, and 9 patients were diagnosed with VAP with CDC criteria. Both criteria have high specificity but poor sensitivity, similar to sensitivity and specificity result in another study [10]. Several causes found to be impacting this number, such as 2 cases of false-positive found in pediatric patients with a genetic disease which requires them to use long term mechanical ventilator through tracheostomy and other several cases in patients with pneumonia as an underlying disease and positive culture result already found prior intubation.
Other false-positive cases were found in patients who gave positive culture results on the day of intubation or less than 2 days post-intubation. On the other side, 1 false-negative case found in CDC criteria was a patient who did not go through sputum or BAL culture examination, so a negative result was assumed for this case. The downfall for this study was the retrospective study which increases the risk of incomplete or missing data and the sample size relatively small.
The PVAP and PNU demographic patients were similar in several characteristics, sex does not have any significant difference in PVAP or PNU group, most samples who developed VAP according to VAE and CDC also relatively had less than 10 in PELOD-2 score, with median PELOD-2 score of 5 and 7.5 respectively. Patients who developed VAP also had a significantly longer duration on a mechanical ventilator, compared to all patients. As consequence, PVAP and PNU will increase the average length of PICU stay of patients, 13.5 days and 12.5 days respectively, compared to 6 days in all samples group. This result is in line with several studies which stated that VAP will increase the morbidity of patients [4,11-13]. Both samples who developed VAP according to VAE and CDC criteria had a longer average duration on a mechanical ventilator (9 days and 11.5 days), compared to 4 days in all samples group.
The other purpose of this study was also to learn the risk factor for VAP. Because VAE had higher sensitivity and specificity, univariate analysis was conducted to assess the association of various categorical risk factors with VAP according to VAE criteria. Results were similar with another study, such as more than 5 days duration on a mechanical ventilator (late-onset) will increase the risk of VAP 7.519 times compared to 2 to 4 days duration on a mechanical ventilator (early-onset) [11,13,14]. Other risk factors were reintubation within 72 hours post-extubation which was increasing the risk to develop VAP as much 4.057 higher. The use of vasoactive drugs and proton pump inhibitors also increased the risk of VAP 4.364 and 5 times more than the sample group who did not receive vasoactive and proton pump inhibitors [15,16]
VAP bundle was also well recognized to be implemented in ICU and PICU to prevent VAP developed in admitted patients. The use of H2 antagonist was one of the key elements in the Adult VAP bundle, specifically as peptic ulcer prophylaxis [17]. However, there are several studies varied between supporting and contradicting the use of H2 antagonist as one of the key elements of the VAP bundle in pediatric patients [18]. Therefore, the most recent study in 2018 which discussed the five-element VAP prevention bundle did not include H2 antagonist as peptic ulcer prophylaxis and one of the key elements in preventing VAP [19]. Our study was in line with this study because the H2 antagonist did not give a significant difference in reducing or increasing VAP.
Patients with genetic syndrome and neuromuscular disease are prone to infection and exposure to the invasive device, as well as longer duration of hospital length of stay compared to the other group. For this reason, genetic syndrome and neuromuscular disease groups were assumed as risk factors of VAP [13]. However, the result of this study did not meet significant differences for this group to be considered as risk factors for a patient developing VAP. Trends of incidence and risk factors of VAP were also found in the immunodeficiency group in adult patients. In contrast, our study did not show any significant difference for this factor presumed as one of the risk factors of VAP. CDC’s new criteria for diagnosing VAP also specifically designed criteria for the immunocompromised group. This is one of the strengths of CDC criteria compared to VAE criteria.
The most number of organisms found in positive culture examination in the VAP group are klebsiella pneumonia and pseudomonas aeruginosa, followed by acinetobacter baumannii. One systematic review in 2021 compared 24 studies which were investigating the most common organism found in VAP patients, and the result also showed gram-negative organisms such as klebsiella pneumonia, pseudomonas aeruginosa, and acinetobacter baumannii are the leading cause of VAP. Those three organisms are the most common organism found in hospital-related or nosocomial infection, in line with VAP which is considered as the second-leading disease that causes HAI.
Conclusion
We compared adult VAE criteria with new CDC criteria in detecting VAP in pediatric patients. New CDC criteria have more specific criteria for several age groups, specific criteria for the immunocompromised group, and patients with pneumonia as an underlying disease. Those specific criteria are supposed to help to diagnose all pediatric patients, prevent excluding some groups and get real number incidence of VAP. The other strength of CDC criteria for the non-immunocompromised group was no invasive examination should be conducted to diagnose VAP, so physicians in rural countries or limited facilities who could not conduct BAL examination, can still diagnose VAP in pediatric patients and treat them faster.
However, CDC still has poor and lower sensitivity compared to adult VAE criteria. Reintubation within 72 hours, longer duration of mechanical ventilation, the use of proton pump inhibitor, and vasoactive drugs should be avoided if not necessarily needed because those are risk factors for pediatric patients developing VAP. A larger study with bigger samples is needed to learn the specificity and sensitivity of the CDC algorithm in diagnosing VAP in pediatric patients. Different study settings, such as prospective studies could be considered to reduce the risk of missing or invalid data. However, this study could help physicians to understand that the negative result of VAP in pediatric patients through the CDC algorithm, does not exclude the possibility of VAP diagnosis.
Acknowledgements
Ethics approval
This is a retrospective study taking data from electronic medical records. No intervention was conducted on subjects and no use of human tissue samples. Approval was obtained by the ethical committee of Universitas Indonesia with reference number: KET-815/UN2.F1/ETIK/PPM.00.02/2019. All methods were performed in accordance with the relevant guidelines and regulations of the Declaration of Helsinki.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding
The authors received no financial support for the research, authorship, or publication of this article.
Authors’ contributions
IY designed the conception, the work and analyzed and interpreted the patient data. SF collected the data. IH collected the data, performed the analysis and drafted the work or substantively revised it. All authors read and approved the final manus.
References
- Foglia E, Meier MD, Elward A. Ventilator-associated pneumonia in neonatal and pediatric intensive care unit patients. Clin Microbiol Rev 2007; 20: 409-425.
- https://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent.pdf
- Alfaray RI, Mahfud MI, Faizun RS. Duration of ventilation support usage and development of ventilator-associated pneumonia: When is the most time at risk? Indonesian Journal of Anesthesiology and Reanimation 2019; 1: 26-31.
- https://aricjournal.biomedcentral.com/articles/10.1186/2047-2994-3-30
- Uçkay I, Ahmed QA, Sax H, et al. Ventilator-associated pneumonia as a quality indicator for patient safety? Clin Infect Dis 2008; 46: 557-563.
- Chang I, Schibler A. Ventilator associated pneumonia in children. Paediatr Respir Rev 2016; 20: 10-16.
- Kumpf O, Braun JP, Brinkmann A, et al. Quality indicators in intensive care medicine for Germany-third edition 2017. Ger Med Sci 2017; 15: Doc10.
- https://www.cdc.gov/nhsn/pdfs/pscmanual/10-vae_final.pdf
- Cirulis MM, Hamele MT, Stockmann CR, et al. Comparison of the new adult ventilator-associated event criteria to the centers for disease control and prevention pediatric ventilator-associated pneumonia definition (PNU2) in a population of pediatric traumatic brain injury patients. Pediatr Crit Care Med 2016; 17: 157-64.
- Wu D, Wu C, Zhang S, et al. Risk factors of ventilator-associated pneumonia in critically ill patients. Front Pharmacol 2019; 10: 482.
- Liu B, Li SQ, Zhang SM, et al. Risk factors of ventilator-associated pneumonia in pediatric intensive care unit: A systematic review and meta-analysis. J Thorac Dis 2013; 5: 525-31.
- Elward AM, Warren DK, Fraser VJ. Ventilator-associated pneumonia in pediatric intensive care unit patients: Risk factors and outcomes. Pediatrics 2002; 109: 758-64.
- Awasthi S, Tahazzul M, Ambast A, et al. Longer duration of mechanical ventilation was foundto be associated with ventilator-associated pneumonia in children aged 1 month to 12 years in India. J Clin Epidemiol 2013; 66: 62-66.
- Vijay G, Mandal A, Sankar J, et al. Ventilator associated pneumonia in pediatric intensive care unit: Incidence, risk factors and etiological agents. Indian J Pediatr 2017; 85(10): 861-866.
- Kusahara DM, Da Cruz C, Avelar AFM, et al. Risk factors for ventilator-associated pneumonia in infants and children: A cross sectional cohor study. American Journal of Critical Care 2014; 23: 6.
- https://www.jscimedcentral.com/EmergencyMedicine/emergencymedicine-2-1027.pdf
- Natale JE, McBeth C, Powne A, et al. Implementation of five-element, inter professional ventilator associated pneumonia prevention budnle in the pediatric intensive care unit. Pediatrics 2018; 141: 118.
- Dananche C, Vanhems P, Machut A, et al. Trends of incidence and risk factors of ventilator-associated pneumonia in elderly patients admitted to french ICUs between 2007 and 2014. Ctricical Care Med 2018; 46: 869-77.
- Kharei S, ist A, Mishra SK. Ventilator-associated pneumonia among ICU patients in WHO Southeast Asian region: A systematic review. PLoS One 2021; 16(3): e0247832.