- Biomedical Research (2007) Volume 18, Issue 2
Dexamethasone antagonizes morphine effects on GSSG levels
Federica Cavallo*, Lucia Micheli, Giorgio Giorgi and Anna CapassoDepartment of Pharmaceutical Sciences, University of Salerno, Italy.
- *Corresponding Author:
- Federica Cavallo
Dipartimento di Scienze Farmaceutiche, Università di Salerno
Via Ponte Don Melillo (84084) Fisciano Salerno, Italy
Accepted date: February 17 2007
Abstract
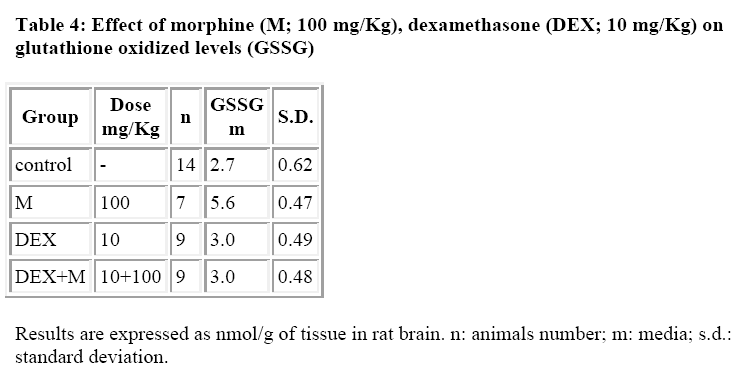
Dexamethasone (DEX) is a highly potent logacting glucocorticoid with negligible sodium retaining properties. Recent evidence has been obtained for an interference caused by DEX on morphine induced analgesia, hypermotility, constipation and contraction of guinea pig isolated ileum. Studies in our laboratory have recently focused on the relationship between morphine and glutathione (GSH) levels. The present study was carried out to determine whether DEX may affect the morphine induced GSH variation in the brain. Male albino Wistar rats (180-200 g) received i.p. morphine (100 mg/kg), DEX (10 mg/kg) and were sacrificed 3 hours after the administration, in the brain GSH and glutathione disulfide (GSSG) were determined by an automatic procedure developed in our laboratory. The results show that DEX is not able to inhibit the GSH levels induced by morphine but completely counteracts the effect on GSSG levels (nmoles/g tissue). In fact, the GSSG for the control group was found to be 2.8+0.56 for the morphine treated group was 5.7+0.46 for the DEX treated group was 3.0+0.49 and for the DEX+Morphine treated group was 3.0+0.48. The present study shows that DEX is also able to interfere with morphine induced GSH variation
Keywords
Dexamethasone, glucocorticoid, analgesia, hypermotility, glutathione disulphide, morphine
Introduction
It is widely reported that hepatic toxicity induced by morphine in rat is related to its metabolites rather than morphine alone [1-7]. These metabolic products are able to induce free radicals and/or binding with glutathione (GSH), the natural scavenger of superoxide radicals.
GSH is one of the most important intracellular thiolic compounds which is involved in several and fundamental cellular functions.
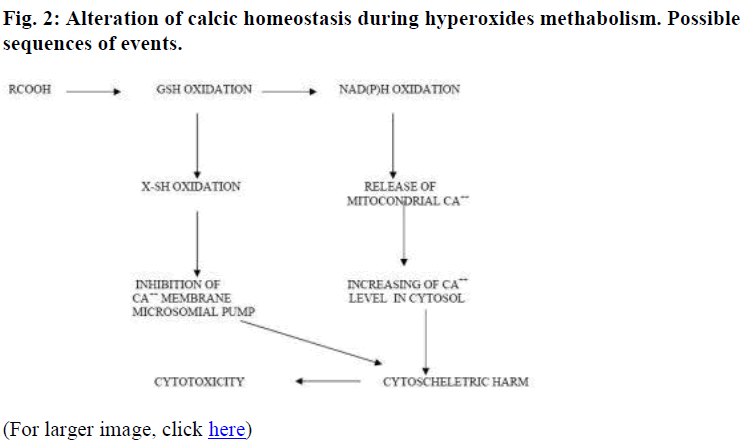
Both GSH conjugation and its subsequent depletion cause a free radicals accumulation as well as morphine metabolites induce directly and indirectly cellular toxicity [8] with enzymatic inactivation, DNA damage and/or lipidic peroxidation (Fig. 1).
In order to verify the presence of such phenomena in brain, we have measured in the rat reduced and oxidized glutathione levels (GSSG) after morphine administration.
Moreover, since it was reported that DEX influences significantly morphine effects [9-13], we have also verified if this glucocorticoid is able to act on morphine-GSH model.
Materials and Methods
For our experiments, we used albino Wistar male rats (average weight 200g) kept in standard conditions of temperature (21°C) and diet (standard diet: MIL, Morini, S.Polo d’Enza, Italy). The animals had free access to water
The animals were divided in 3 groups which received through i.p.:
• a solution of morphine 15,20, 50 and 100 mg/Kg;
• a solution of DEX 10 mg/Kg;
• concurrent administration of morphine 100 mg/Kg and DEX 10 mg/Kg.
The control group which received through i.p. administration only saline solution in the same quantity of the corresponding group of rats which didn’t have any treatment were considered “white”.
Animals were sacrificed by decapitation always at the same hour of the day (4 P.M) to avoid the influence of circadian rhythm for GSH determination. After sacrifice, the brain was quickly removed and immediately dissected on a cold surface (0°C) and homogenized at pH=4 phosphate buffer (1:3 w/v).
Homogenates were used to determine:
• GSH by the Tietze’s method, by a procedure autmated in our laboratory [15];
• GSSG with Griffith method [16];
• Proteins in accordance with Lowry method [17]
No statistical differences were observed among the control groups and therefore they were collected in a single pool.
Results and Discussion
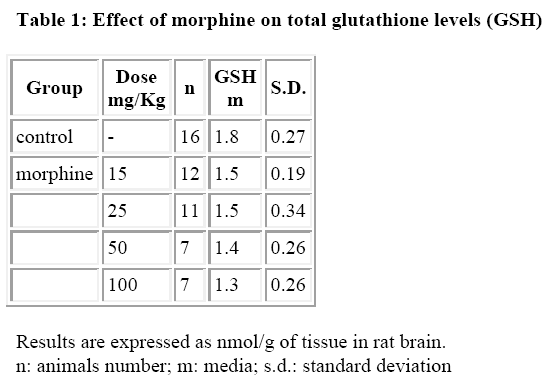
The results are reported in Tables 1-4. Morphine at the doses used (15, 20, 50 and 100 mg/Kg/ip) did not induce per se a significative variation of total GSH in the rat brain (Table 1).
This observation may appear in contrast with previous data obtained with hepatic GSH [18] where total GSH depletion is significant and dose dependent. This result may be explained considering that the reactive metabolites do occur only in the liver for oxidation by cytocrome P450 and that they are not transported in the brain (or only partially). Therefore, the content of total GSH could not receive a diminution proportional to the dose of morphine administered, considering also the variability of its metabolism [19].
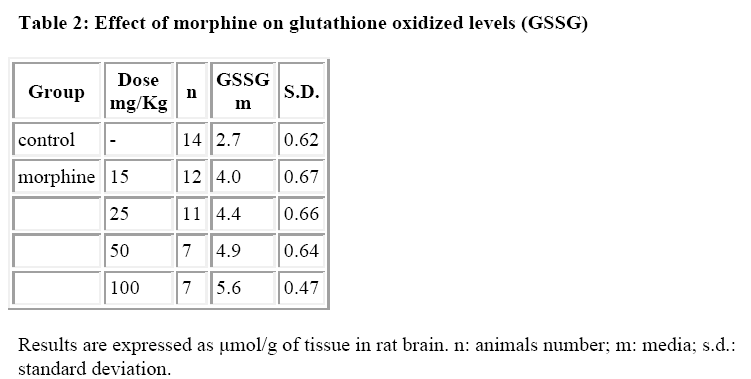
By contrast, it was observed that morphine increased significantly and dose-dependently the GSSG levels (Table 2).
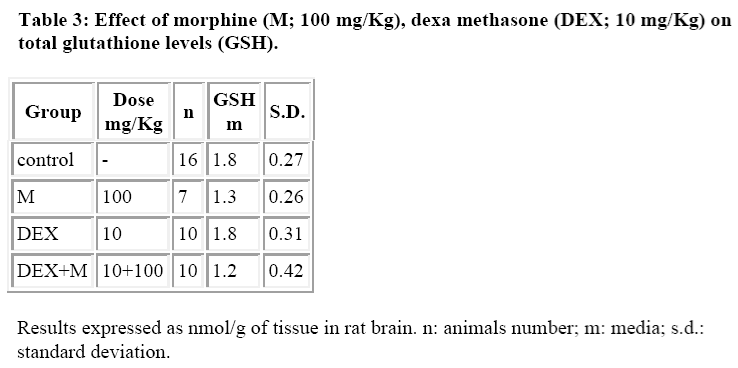
DEX alone did not induce any significant alterations both in GSH and GSSG levels (Tables 3 and 4) whereas it was able to reduce the morphine induced increase in GSSG levels (Table 4).
The results of the present study show a futher interaction between DEX and morphine thus confirming our previous studies [8-12]. Although, the mechanism underlying this interaction is still unclear, we may suggest a hypothesis. One possibility may be due to a mechanism linked to Ca2+ homeostasis. It is known that glucocorticoids increase the uptake of Ca2+, establishing the altered balance caused by the increase of intracellular Ca2+ after morphine administration [21].
The return in Ca2+ balanced conditions and in the consequent regular membrane permeability may have a key role in the modulation of “oxidative stress” shown by the abnormal enhance of brain GSSG levels found after morphine administration (100 mg/kg i.p.).
These results may also explain intracellular Ca2+ increase, with immediately hyperpolarization of cellular membrane, showed after morphine administration [21].
Also, we observed a significative increased of GSSG/GSH ratio with a corresponding reduction of NADPH levels and a modification of Ca2+ compartmentalization (Figure 2) [20-22].
References
- Todaka T, Ishida T, Kita H, Narimatsu S, Yamano S.Bioactivation of morphine in human liver: isolation and identification of morphinone, a toxic metabolite. Biol Pharm Bull 2005; 28: 1275-1280.
- Jairaj M, Watson DG, Grant MH, Skellern GG. The toxicity of opiates and their metabolites in HepG2 cells. Chem Biol Interact 2003, 146: 121-129.
- Toki S, Yamano S. Production of morphinone as a metabolite of morphine and its physiological role. J.Pharmacol Exp Ther 1999; 119: 249-267.
- Pavabvash S, Beheshtian A, Salmasi AH, Kiumehr S, Ghahremani MH, Tavangar SM, Sabzevari O, Dehpour AR. Chronic morphine treatment induces oxidant and apoptotic damage in the mice liver. Life Sci 2006, 79: 972-980.
- Atici S, Cinel I, Cinel L, Doruk N, Eskandari G, Oral U. Liver and kidney toxicity in chronic use of opioids: an experimental long term treatment model. J Biosci 2005; 30: 245-52.
- Misra AL., Vadlamani NL, Pontani RB, Mule SJ. Oxidative damage of biomolecules in mouse liver induced by morphine and protected by antioxidants. Basic Clin Pharmacol Toxicol 2004; 95: 53-58.
- William S, Sekar M, Subramanian S, Govindasamy S. Toxic effect of morphine and the antagonistic role of naloxone on isolated rat hepatocytes. Biochem Int 1991; 23: 1071-1077.
- Calignano A, Capasso A, Persico P, Mancuso F, Sorrentino L. Dexamethasone modifies morphine-, atropine-, verapamil induced constipation in mice. Gen Pharmacol 1992; 23: 753-756.
- Persico P, Capasso A, Calignano A, Sorrentino L. Effect of RU-38486 on dexamethasone reversal of morphine-induced constipation in mice. Gen Pharma-col 1991; 22: 867-868..
- Capasso A., Di Giannuario A, Loizzo A, Pieretti S, Sorrentino L. Dexamethasone induces biphasic effect on morphine hypermotility in mice: a dose-related phenomenon. Life Sci 1991; 49: 1411-1418.
- Pieretti S, Capasso A, Di Giannuario A, Loizzo A, Sorrentino L. The interaction of peripherally and centrally administered dexamethasone and RU 38486 on morphine analgesia in mice. Gen Pharmacol 1991; 22: 929-933..
- Capasso A., Pieretti S, Di Giannuario A, Loizzo A, Sorrentino L. Time and dose related influence of dexamethasone on morphine-induced hypermotility in mice. Agents Actions 1992; C121-3.
- Williams DB. Toxicophores: investigations in drug safety. Toxicology 2006; 226: 1-11.
- Giorgi G. Micheli L and Segre G. A diurnal variation of the glutathione content in rat level and stomach. IRCS Med Sci 1984; 12: 560-561.
- Giorgi G. And Micheli L. Automation of spectrophotometric measurement. Int Lab 1987; 17: 52-55
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinlypyridine. Anal Biochem 1980; 106: 207-212.
- Lowry OH., Farr NJ, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193: 265-272
- Micheli L. Atti del III Convegno Nazionale “Giovani Cultori di Neuroscienze”. Firenze 28-30 Novembre 1991, P 105-122.
- Shang GW, Liu DN, Yan LH, Cui XY, Zhang KP, Qi C, Chen J.. Nociceptive stimulus modality-related difference in pharmacokinetic-pharmacodynamic modeling of morphine in the rat. Pharmacol Biochem Behav 2006; 85: 464-73
- Reed DJ, Fariss MW. Glutathione depletion and susceptibility. Pharmacol Rev 1984; 36: 25S-33S
- Smith FL, Dombrowski DS, Dewey WL. Involvement of intracellular calcium in morphine tolerance in mice. Pharmacol Biochem Behav 1999; 62: 381-388
- Karbach U. Segmental heterogeneity of cellular and paracellular calcium transport across the rat duodenum and jejunum. Gastroenterology. 1991; 100: 47-58.