Brief Report - Current Trends in Cardiology (2022) Volume 6, Issue 4
Development and evaluation of a new artificial intelligence model for the analysis of electrocardiographic abnormalities.
Diandro Marinho Mota1, Fabiano Barcellos Filho2, Amanda Bergamo Mazetto2, Marlon Woelffel Candoti2, José Henrique Lopes2
1Department of Cardiology, Dante Pazzanese Institute of Cardiology -500 Dr. Dante Pazzanese Avenue, São Paulo, SP 04012-909, Brazil
2Department of Cardiology, Neomed Healthtech, Alameda Vicente Pinzon 54 - Vila Olimpia, Sao Paulo, Brazil
- Corresponding Author:
- Diandro Mota
Department of Cardiology
Dante Pazzanese Institute of Cardiology -500 Dr. Dante Pazzanese Avenue
São Paulo, SP 04012-909, Brazil
E-mail: - diandro@me.com
Received: 24-May-2022, Manuscript No. AACC-22-62034; Editor assigned: 26-May-2022, Pre QC No. AACC-22-62034 (PQ); Reviewed: 09-June-2022, QC No AACC-22-62034;Revised: 13-June-2022, Manuscript No. AACC-22-62034(R); Published: 20- June-2022, DOI:10.35841/aacc-6.4.116
Citation: Mota DM, Filho FB, Mazetto BA, et al. Development and evaluation of a new artificial intelligence model for the analysis of electrocardiographic abnormalities. Curr Trend Cardiol. 2022;6(4):116
Abstract
Cardiovascular disease has reached alarming numbers in the last few years. However, there was always a need for technologies that help in the diagnosis and screening of diseases, but there is still a need to increase confidence in Artificial Intelligence (AI) models. Research with signal extraction and processing has high accuracy in detecting cardiac anomalies, although Deep Learning models have been better. Our objective is to develop an AI model to detect high sensitivity anomalies compared to medical reports. We used image processing with signal extraction and processing from digitized electrocardiograms using the rules of the Brazilian Directive on Analysis and Emission of Electrocardiograms. The AI model scored 96% on sensitivity and 26.9% on specificity, with an F1 of 0.83, resulting in a great AI for case screening. We conclude that AI models, in which we use the ECG standards for classification, can be included in the arsenal of predictive methods for screening with high sensitivity and bring interpretability to complement the most efficient algorithms.
Keywords
Artificial intelligence, Deep learning, Machine learning, Electrocardiogram.
Introduction
The number of cases of cardiovascular diseases (CVDs) has nearly doubled during the last 30 years, a raise from 271 million cases in 1990 to 523 million cases in 2019, which has directly and increasingly impacted the number of CVDrelated deaths, from 12.1 million in 1990 adding up to 18.6 million in 2019 [1]. Article publication related to the use of artificial intelligence (AI) aimed at clinical decision support, electrocardiogram (ECG) classification and cardiovascular anomalies diagnosis has heightened in the latest years, leading the scientific community to gather intervention-intended clinical evidence resourcing to this technology in clinical trials. Studies in cardiology are mostly focused on binary classification to distinguish between normal and abnormal heartbeat, normal and premature ventricular beats (PVBs), normal and diseased beats. Other studies additionally follow labeling rule classification recommendations, such as AAMI standards (normal, supra-ventricular, ventricular, fusion and unknown beats).
In most studies, signal processing is made directly on the machine, taking ECG recordings into account, which allows for the identification of time-dependent abnormalities, such as heartbeat morphology alterations in time or heartbeat rate alterations. Following the best international-standard guidelines and their recommended practices is extremely important for medical software validation, mainly the ones involving the use of AI algorithms, which is essential to guaranteeing both safety and effectiveness.
Safety and effectiveness analysis is imperative to the validation process of health intervention propositions. Regarding medical software’s, the aforesaid process can be guaranteed by acknowledging the best guidelines put forth by international scientific societies, such as TRIPOD-AI and PROBAST-AI, in order to evaluate the study quality and interpret its findings, potentially reducing research waste. In this context, the purpose of this paper is to detect with high sensitivity abnormalities on the electrocardiogram, in comparison to reports by specialized physicians. With this, we look forward to developing an AI system that improves screening of patients with potential electrocardiogram-traceable cardiovascular anomalies.
Methods
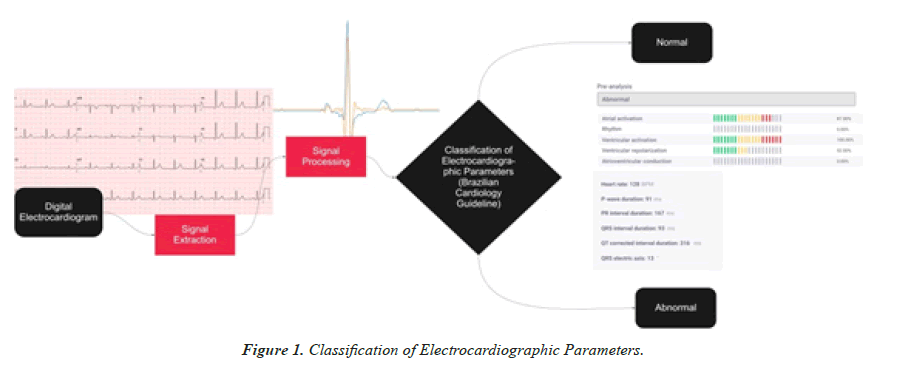
It is proposed to create an AI Intelligence with the ability to detect anomalies in ECGs. This performance is based on 12-lead ECG images, in PDF format, from which signals are extracted. The collected data are stored in the cloud, processed in image format and analyzed from the signals. The extraction takes place through the Open CV and PIL libraries for image processing and scipy for signal processing, with the signal and pywt packages for processing the waves with the Wavelet function, and Scikit- Learn, where linear regression methods were used.
Using the processed signals, measurements related to five ECG parameters are evaluated: rhythm, atrial depolarization, atrioventricular conduction, ventricular depolarization and ventricular repolarization. Each measurement obtained from these cardiac cycle phases, recorded in the exam, is compared with the standard values according to the Guidelines of the Brazilian Society of Cardiology on Analysis and Issuance of Electrocardiographic Reports III. The probability of alteration in each of the parameters is shown according to the presence of anomalies detected by the AI (Figure 1).
The AI validation was made from a retrospective, cross-sectional study, with the collection of ECG data from the admission of patients to the Octopus platform, at Neomed's partner hospitals and clinics. This platform was developed to enable the analysis of ECGs from different manufacturers in the same place, working in the SaaS (software as a service) standard.
Regarding data analysis, the reports issued by specialists on the Octopus platform were first divided into two groups: “normal” and “abnormal”, according to the Guideline. From then on, sensitivity, specificity, precision, positive predictive value, negative predictive value and the area under the receiver operating characteristic curve (AUC) were evaluated for the best performing models. We have calculated the positive predictive value and negative predictive value for all possible cohort prevalence values and illustrated the relationship between them for the AI model. All analyzes were performed in Python 3.7 language.
Results
The study sample consisted of 3,501 patients: 2,387 (68.16%) with anomalies and 1,115 with no anomalies. 1792 of the patients were women aged 58.9 on average and 1709 were men aged 56.9 on average, as shown in Table 1. Artificial Intelligence was able to detect 3,502 true negative events and 300 true positive ones. 96% sensitivity obtained and 26.9% specificity, with a 0.83 F1, enacting AI as an important case screening asset.
| Electrocardiogram Classification | |||
|---|---|---|---|
| Characteristic | Normal | Abnormal | Total |
| Baseline and demographics | 1,115 | 2,386 | 3,501 |
| Male – n (%) | 496 (44.48%) | 1,213 (50.83%) | 1.709 (48.81%) |
| Female – (%) | 619 (55.51%) | 1,173 (49.16%) | 1.792 (51.18%) |
| Age (Years) | 49.08 | 62.04 | 57.92 |
| ECG abnormalities – n (%) | |||
| Non-specific left ventricular repolarization abnormalities | 502 (21,03%) | - | |
| Atrial fibrillation | 217 (9.09%) | - | |
| Sinus tachycardia | 157 (6.58%) | - | |
| Left ventricular overload | 165 (6.91%) | ||
| AMI with SST | 105 (4.40%) | ||
| Left anterior superior divisional block | 100 (4.19%) | ||
| Left bundle branch block | 99 (4.14%) | ||
| Right bundle branch block | 91 (3.81%) | ||
| Right bundle branch conduction disorder | 80 (3.35%) | ||
| Electrically inactive area | 72 (3.01%) | ||
| Others | 798 (33.44%) | ||
Table 1. Clinical and demographic characteristics related to the study electrocardiograms.
The study observed situations in which the AI points out ECG tracing changes not covered by the reported exam. A second analysis, more detailed and optimized by exam scanning, led to the conclusion that AI boosts a very high sensitivity to small-change detection, which can go unnoticed by the human eye, thus improving the identification potential of discrete changes which might go unnoticed by the specialist physician.
Discussion
The premise of this work is to use signal processing as a basis for carrying out ECG-tracing analyses that simulate the physician's work when reporting according to the guideline. The delimitation of specific signal points can be done with Wavelet to reliably outline P, QRS and T wave points as done in the work of Di Marco [2,3]. Despite the advances in Deep Learning, classic models that extract features with signal processing and compare them with cardiological guidelines, as does the model used in our work, show performance comparable and sometimes superior to robust Deep Learning models are given below.
Bachtiger [2], in their recent work on deep neural networks, they obtained 91.9% sensitivity in a database comprising 35,970 individuals. Jo [3] as machine learning models obtained 94.7% sensitivity in an 86,802-patient dataset. Studies such as the one by Bachtiger [2] highlight a single lead perspective instead of a 12-lead perspective, as endorsed by the present work. Likewise, Zhang [5] demonstrate that single lead models show lower performance compared to the use of all 12 leads simultaneously. Other works such as those by Teplitzky et al, [4]. prove superior performance, yet distinguished by the direct use of signals, differing in production from the one this work presents. Signal extraction by digital image processing produces noise which hinders anomaly classification.
Outlining studies with clinical trials, which go a step beyond an external algorithm validation, is a significant aspect to generating cutting-edge real-life clinic evidence using Artificial Intelligence. Therefore, new studies need to be carried out and validated, given that few cardiology studies regard AI as an intervention, as per the review by Topol presenting the new AI healthcare guidelines.
Conclusion
Starting at the development of a digital roadmap for the patient, exam follow-up by the doctor needs trustable ECG interpretation algorithms. Previous to sophisticated, machinelearning- based models, it is critical to have interpretable ECG wave measurements models at hand, given that interpretability leads to more reliable algorithms. By using double processing data and ECG images and extracting their signals, Artificial Intelligence was able to screen positive cases with high sensitivity, employing simpler yet highexplainable algorithms. Medical guideline-based Artificial Intelligence fills a missing gap in the development of ECG algorithmic interpretation and could lever new Machine Learning technologies to advance as well.
References
- `Cardiovascular Diseases - PAHO/WHO – PAHO. Paho.org. 2022.
- Bachtiger P, Petri CF, Scott FE, et al. Point-of-care screening for heart failure with reduced ejection fraction using artificial intelligence during ECG-enabled stethoscope examination in London, UK: a prospective, observational, multicenter study. Lancet Digit Health. 2022;4(2):e117-e125.
- Jo YY, Cho Y, Lee SY, et al. Explainable artificial intelligence to detect atrial fibrillation using electrocardiogram. Int J Cardiol. 2021;328:104-110.
- Teplitzky BA, McRoberts M, Ghanbari H. Deep learning for comprehensive ECG annotation. Heart Rhythm. 2020;17(5 Pt B):881-888.
- Zhang D, Yang S, Yuan X, et al. Interpretable deep learning for automatic diagnosis of 12-lead electrocardiogram. iScience. 2021;24(4):102373.
Indexed at, Google Scholar, Cross ref
Indexed at, Google Scholar, Cross ref
Indexed at, Google Scholar, Cross ref