Research Article - Biomedical Research (2017) Volume 28, Issue 20
Determination of amygdalin in the fruit of Eriobotrya japonica Lindl by high performance liquid chromatography
Hui Wu1, Chao Cao2 and Chunhua Zhou2,3*
1Experimental Farm of Yangzhou University, Yangzhou, Jiangsu, PR China
2College of Horticulture and Plant Protection, Jiangsu Key Laboratory of Crop Genetics and Physiology, Yangzhou University, Yangzhou, Jiangsu, PR China
3Joint International Research Laboratory of Agriculture and Agri-Product Safety, Yangzhou University, Yangzhou, Jiangsu, PR China
- *Corresponding Author:
- Chunhua Zhou
College of Horticulture and Plant Protection
Yangzhou University, PR China
Accepted date: October 3, 2017
Abstract
Amygdalin is widely distributed in loquat plant which belongs to Rosaceae and used as an important pharmaceutical compound. In this research, High Performance Liquid Chromatography (HPLC) was used to determine the content of amygdalin in different tissues of loquat fruit cv. ‘Dahongpao’, as well as leaves, roots and flowers. The results demonstrated that the kernel had the highest content of amygdalin among different fruit tissues, while the fruits flesh had the lowest content. Moreover, results showed significant differences in the content of amygdalin between kernel and leaves, while there were no significant differences between the content of kernel compared to roots and flowers. On the basis of above study, the content of amygdalin was determined in the kernel during different developmental stages and different cultivars. Results showed significant difference of the content of amygdalin in the kernel during different developmental stages and different cultivars. The results of this study can provide a theoretical basis for the comprehensive utilization of loquat fruit processing waste in the future.
Keywords
Loquat, Amygdalin, High performance liquid chromatography
Introduction
Amygdalin, belongs to one of the cyanogenic glucosides (Figure 1), is widely distributed in the plant kingdom, especially in the seeds of Rosaceae plant with higher content than those of non-Rosaceae species [1-4]. The molecular formula is C20H27NO11, consisting of one unit of Hydrocyanic acid (HCN), one unit of benzaldehyde and two units of glucose. Amygdalin itself is non-toxic, but its production HCN decomposed by some enzymes is poisonous substance, which has some protective effects on plants [5]. Pharmacological studies demonstrated that amygdalin has many bioactivities such as renal fibrosis and angiogenesis inhibition [6,7], endocrine regulators [8], anti-atherosclerotic, gastroprotective and immunomodulatory effects [9-11], neuroprotective and neuritogenic activities [12]. As a component of antitumor and anticancer drug, amygdalin also has increasingly attracted many people's attention [13-19].
At present, the studies on the extraction, identification and pharmacological effects of amygdalin were mainly focused on the kernel of Prunus amygdalus [20-23], Prunus armeniaca [24-28], Prunus persica [29] and Prunus salicina [30], with few concerning on the fruit of Eriobotrya japonica [31,32]. The objective of this study is to determine the content of amygdalin in loquat fruit by High Performance Liquid Chromatography (HPLC).We anticipate that the information obtained in this research can serve as foundation of future studies to provide a theoretical basis for the evaluation of the health effects of loquat fruit and comprehensive utilization of waste materials during loquat fruit processing.
Materials and Methods
Plant materials and chemicals
Fruits of loquat cv. ‘Dahongpao’ were obtained from Tangxi Xitaiyang Loquat Production Co. Ltd. (Hangzhou, Zhejiang Province, P.R. China) at ripe stage and were used to compare the content of amygdalin between different fruit tissues (i.e. peduncle, peel, flesh, seed coat and kernel) as well as other organs such as leaves, roots and flowers. On the other hand, fruits of two cultivars, namely ‘Ruantiaobaisha’ (Whitefleshed) and ‘Dayeyangdun’ (Red-fleshed), were obtained from the orchard of Yuhang Loquat Science Institute (Hangzhou, Zhejiang Province, P.R. China) during six different developmental stages including ripe stage. 20~100 fruits were picked depending on fruit size and weight. Fruits were harvested in 7 April, 17 April, 27 April, 7 May, 17 May and 24 May, respectively and the content of amygdalin in the kernel was determine. Moreover, loquat fruit of twenty-four cultivars, including 11 White-fleshed cultivars (‘Baisha’), namely ‘90-1’, ‘Baiyu’, ‘Bingtangzhong’, ‘Dazhong’, ‘Guanyu’, ‘Jidanbai’, ‘Luqiaobaisha’, ‘Qingzhong’, ‘Ruantiaobaisha’, ‘Tianzhong’, ‘Tongpi, and 13 Red-fleshed cultivars (‘Hongsha’), namely ‘90-2’, ‘Algeie’, ‘Baozhu’, ‘Bahong’, ‘Dahongpao I’, ‘Dahongpao II’, ‘Dayeyangdun’, ‘Jiajiao’, ‘Jidanhong’, ‘Luoyangqing’, ‘Marc’, ‘Pelusheis’, ‘Zaozhong’, were used to compare the level of amygdalin in the kernel. Ripe fruits were obtained from the orchard of Yuhang Loquat Science Institute (Hangzhou, Zhejiang Province, P.R. China) and Taihu Technological Popularization Center of Evergreen Fruit (Suzhou, Jiangsu Province, P.R. China). Twenty fruits of each cultivar were collected from loquat trees and used as plant material. After transportation to the laboratory, fruits were separated into peel, flesh and kernel, and then all the tissues were cut into small pieces. Different organs and tissues were dried in the oven until reached unchangeable weight, then grounded into powder and stored at -20°C until analysis.
Amygdalin standard compound was obtained from Sigma Chemical Company (St. Louis, MO, USA). HPLC grade methanol was purchased from Caledon Company (Georgetown Ont., Canada). All the other reagents used in this experiment were of analytical grade.
Preparation of standard and extract solutions for determination of amygdalin
0.5 g of different organs and tissues were transferred into a conical flask, and soaked with 20 ml ethanol for 2 h followed by ultrasonic extraction with TBT/C-YCL 500 Tt/3P (D) for 30 min using ultrasonic extraction machine (Sinobest electronic Co. Ltd., Jining, Shandong Province, P.R. China). The samples were extracted twice under the same condition, and filter fluid of both extractions were combined and evaporate till dryness at 35°C. The residue was dissolved in 1 ml HPLC grade methanol and transferred into a 1.5 ml Eppendorf tube. The crude extraction was filtered through a 0.30 μm micro-filter before HPLC analysis.
A stock solution of amygdalin standard compound was prepared with HPLC grade methanol. A series of five different standard dilutions containing 50, 100, 200, 300, 400 μg/ml were obtained. For each concentration, 20 μL solution was injected into HPLC, and used to plot amygdalin standard curve which was constructed according to the peak area and the concentration of amygdalin.
Determination of amygdalin and quantification
Determination of amygdalin was performed according to the method of Zhou et al. [32] using a HPLC system (Beckman, USA) equipped with 125 pump, 166 UV detector and an ODS C18 column (250 mm × 4.6 mm, 5 μm). The compound was detected at 210 nm at room temperature with an eluent flow rate of 1.0 ml/min. The mobile phase consisted of methanol (A) and 0.03 mol/L phosphate buffer (pH 2.8) (B) with a ratio of 25:75 (A:B, v/v). The quantification of the content of amygdalin in different plant materials were calculated using the standard curve.
Statistical analysis
Standard Deviations (SD) were calculated by Origin (Microcal Software Inc., Northampton, MA, USA). Means were separated using Duncan’s new multiple range test (DPS version 3.11).
Results and Discussion
Content of amygdalin in different organs and tissues of loquat
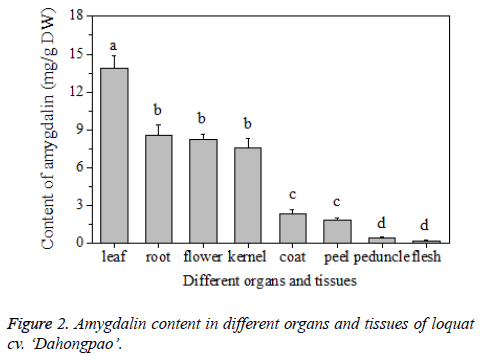
Results showed that the content of amygdalin varied in different organs and tissues of loquat fruit (Figure 2). Among different organs, the leaves had the highest content of amygdalin, of 13.86 ± 1.03 mg/g, while the flower and root were found containing similar amount of amygdalin, with the level of 8.58 ± 0.85 mg/g and 8.24 ± 0.44 mg/g, respectively, Among different tissues of loquat fruit, the content of amygdalin also demonstrated great variation. The kernel showed the highest content of 7.58 ± 0.76 mg/g, which was slightly lower than the level in the flowers or roots. While the fruit flesh had the lowest content of 0.20 ± 0.02 mg/g. Zhao et al. [2] found that the content of amygdalin in the kernel of apricot, peach, plum and bitter apricot was much higher than that in pulps, roots, shoots or leaves. In this study, the highest content of amygdalin occurred in leaves of loquat, while the kernel contained the highest content compared to other fruit tissues such as coat, peel, peduncle and flesh, Therefore, according to the above result, the content of amygdalin was only analysed in the kernel of fruit during different development stages and in different cultivars of loquat.
Content of amygdalin in the kernel during different developmental stages
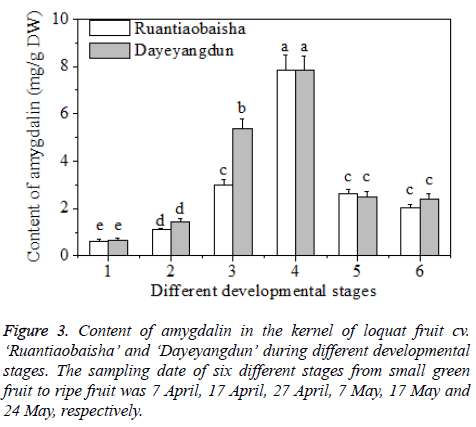
The content of amygdalin in the kernel of the cultivars of ‘Dayeyangdun’ and ‘Ruantiaobaisha’ varied considerably during fruit development. Results showed that the content increased gradually in early stage, and reached the highest value in Stage 4, then decreased sharply, and maintained at steady level till fruit ripening (Figure 3). Zhao et al. [2] investigated the amygdalin content of four species of stone fruit trees during different stages, and found that the amygdalin level in kernels constantly increased and reached the highest value at last stage. Our results were inconsistent with above studies. Maybe amygdalin contents during fruit development differ in different fruit species. Amygdalin biosynthesis requires the participation of sugar, so the peak appearance of amygdalin may be related to sugar biosynthesis, while the decreased of amygdalin in the kernel maybe resulted from accumulation of soluble sugar in the fruit, especially in the flesh tissue.
Content of amygdalin in the kernels of different cultivars
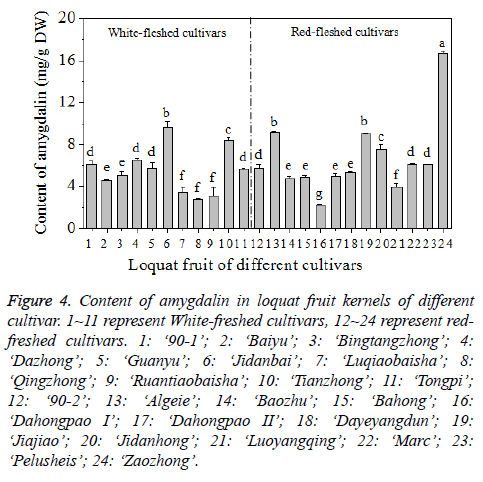
The content of amygdalin in the kernel varied due to different loquat cultivars. The content ranged between 2.44 and 16.70 mg/g, with the average level of 6.16 ± 2.99 mg/g (Figure 4). The differences between the highest and lowest amygdalin content among different cultivars was 6.84 fold, the difference is much higher than those of oleanolic and ursolic acid contents in loquat fruit peel [33]. Among the 24 cultivars analysed, ‘Dahongpao I’ had the lowest content of amygdalin in the kernel of 2.24 ± 0.11 mg/g, while ‘Zaozhong’ had the highest content of 16.70 ± 0.21 mg/g. In this study, the content of amygdalin in the kernel of loquat fruit is similar to the values reported for the seeds of some Rosaceae species (0.12 mg/ g-17.49 mg/g) and much higher than the values of the seeds of non-Rosaceae (0.01-0.21 mg/g) and processed products (n.d.-0.12 mg/g) [3]. Moreover, the content of amygdalin of the kernel of loquat fruit is higher than that of the kernels from 15 commercial almond cultivars which range from 0.443 to 1.866 mg/g and from 0.250 mg/g to 2.200 mg/g for two consecutive seasons, respectively [24], and also higher than that of the seeds from 15 varieties of apples with the levels ranged from 1 mg/g to 4 mg/g [4].
Figure 4: Content of amygdalin in loquat fruit kernels of different cultivar. 1~11 represent White-freshed cultivars, 12~24 represent redfreshed cultivars. 1: ‘90-1’; 2: ‘Baiyu’; 3: ‘Bingtangzhong’; 4: ‘Dazhong’; 5: ‘Guanyu’; 6: ‘Jidanbai’; 7: ‘Luqiaobaisha’; 8: ‘Qingzhong’; 9: ‘Ruantiaobaisha’; 10: ‘Tianzhong’; 11: ‘Tongpi’; 12: ‘90-2’; 13: ‘Algeie’; 14: ‘Baozhu’; 15: ‘Bahong’; 16: ‘Dahongpao I’; 17: ‘Dahongpao II’; 18: ‘Dayeyangdun’; 19: ‘Jiajiao’; 20: ‘Jidanhong’; 21: ‘Luoyangqing’; 22: ‘Marc’; 23: ‘Pelusheis’; 24: ‘Zaozhong’.
Conclusions
In loquat fruit, amygdalin was enriched in the kernel. The amygdalin level in fruit kernel was similar to that in the flowers or roots, and was significantly lower than that in the leaves, while the flesh contained trace amount of amygdalin. Furthermore, the amygdalin content in the kernel varied considerably during different developmental stages and in different cultivars of loquat. The results can provide a theoretical basis for the comprehensive utilization of loquat fruit processing waste in the future.
Acknowledgements
We are grateful to Yuhang Loquat Science Institute, Tangxi Xitaiyang Loquat Production Co. Ltd., and Taihu Technological Popularization Center of Evergreen Fruit of Jiangsu Province for kindly providing the plant materials used in this research. Thanks Dr Muna A.M. El Hadi of Gezira University in Sudan for helping us to improve this manuscript. This research was financially supported by the National Natural Science Foundation of China (Grant no. 31171934), the Priority Academic Program Development from Jiangsu Government and Excellent Youth Backbone Teachers of Yangzhou University (2014).
References
- Ganjewala D, Kumar S, Asha DS, Ambika K. Advances in cyanogenic glycosides biosynthesis and analyses in plants: A review. Acta Biol Szeged 2010; 54: 1-14.
- Zhao YY. Amygdalin content in four stone fruit species at different developmental stages. Sci Asia 2010; 38: 1513-1874.
- Bolarinwa IF, Orfila C, Morgan MR. Amygdalin content of seeds, kernels and food products commercially-available in the UK. Food Chem 2014; 152: 133-139.
- Bolarinwa IF, Orfila C, Morgan MR. Determination of amygdalin in apple seeds, fresh apples and processed apple juices. Food Chem 2015; 170: 437-442.
- Swain E, Poulton JE. Utilization of Amygdalin during Seedling Development of Prunus serotina. Plant Physiol 1994; 106: 437-445.
- Mirmiranpour H, Khaghani S, Zandieh A, Khalilzadeh OO, Gerayesh-Nejad S, Morteza A, Esteghamati A. Amygdalin inhibits angiogenesis in the cultured endothelial cells of diabetic rats. Indian J Pathol Microbiol 2012; 55: 211-214.
- Guo J, Wu W, Sheng M, Yang S, Tan J. Amygdalin inhibits renal fibrosis in chronic kidney disease. Mol Med Rep 2013; 7: 1453-1457.
- Halenar M, Chrastinova L, Ondruska L, Jurcík R, Zbynovska K, Tušimová E, Kovacik A, Kolesarova A. The evaluation of endocrine regulators after intramuscular and oral application of cyanogenic glycoside amygdalin in rabbits. Biologia 2017; 72: 468-474.
- Baroni A, Paoletti I, Greco R, Satriano RA, Ruocco E, Tufano MA, Perez JJ. Immunomodulatory effects of a set of amygdalin analogues on human keratinocyte cells. Exp Dermatol 2005; 14: 854-859.
- Nabavizadeh F, Alizadeh AM, adroleslami ZS, Adeli S. Gastroprotective effects of amygdalin on experimental gastric ulcer: Role of NO and TNF-alpha. J Med Plants Res 2011; 5: 3122-3127.
- Deng JG, Wang HL, Liu YD, Li CY, Hao EW, Du ZC, Bao CH, Lv JZ, Wang Y. Anti-atherosclerotic effects mediated by the combination of probucol and amygdalin in apolipoprotein E-knockout mice fed with a high fat diet. J Anim Vet Adv 2012; 11: 20-25.
- Cheng Y, Yang C, Zhao J, Tse HF, Rong J. Proteomic identification of calcium-binding chaperone calreticulin as a potential mediator for the neuroprotective and neuritogenic activities of fruit-derived glycoside amygdalin. J Nutr Biochem 2015; 26: 146-154.
- Fukuda T, Ito H, Mukainaka T, Tokuda H, Nishino H. Anti-tumor promoting effect of glycosides from Prunus persica seeds. Biol Pharm Bull 2003; 26: 271-273.
- Kwon HY, Hong SP, Hahn DH, Kim JH. Apoptosis induction of Persicae Semen extract in human promyelocytic leukemia (HL-60) cells. Arch Pharm Res 2003; 26: 157-161.
- Park HJ, Yoon SH, Han LS, Zheng LT, Jung KH, Uhm YK, Lee JH, Jeong JS, Joo WS, Yim SV, Chung JH, Hong SP. Amygdalin inhibits genes related to cell cycle in SNU-C4 human colon cancer cells. World J Gastroenterol 2015; 11: 5156-5161.
- Zhou CS, Qian LC, Ma HL, Yu XJ, Zhang YZ, Qu WJ, Zhang XX, Xia W. Enhancement of amygdalin activated with beta-D-glucosidase on HepG2 cells proliferation and apoptosis. Carbohyd Polym 2012; 90: 516-523.
- Chen Y, Ma JS, Wang F, Hu J, Cui A, Wei CG, Yang Q, Li F. Amygdalin induces apoptosis in human cervical cancer cell line HeLa cells. Immunopharmacol Immunotoxicol 2013; 35: 43-51.
- Makarevic J, Tsaur I, Juengel E, Borgmann H, Nelson K, Thomas C, Bartsch G, Haferkamp A, Blaheta RA. Amygdalin delays cell cycle progression and blocks growth of prostate cancer cells in vitro. Life Sci 2016; 147: 137-142.
- Todorova A, Pesheva M, Iliev I, Bardarov K, Todorova T. Antimutagenic, antirecombinogenic, and antitumor effect of amygdalin in a yeast cell-based test and mammalian cell lines. J Med Food 2017; 20: 360-366.
- Dicenta F, Martinez-Gomez P, Grane N, Martin ML, Leon A, Canovas JA, Berenguer V. Relationship between cyanogenic compounds in kernels, leaves, and roots of sweet and bitter kernelled almonds. J Agric Food Chem 2002; 50: 2149-2152.
- Ferrara G, Maggio P, Pizzigallo MDR. Cyanogenic D-amygdalin contents of the kernels of cultivated almonds and wild Amygdalus webbii Spach. J Hort Sci Biotechnol 2010; 85: 410-414.
- Yildirim AN, San B, Koyuncu F, Yildirim F. Variability of phenolics, alpha-tocopherol and amygdalin contents of selected almond (Prunus amygdalus Batsch.) genotypes. J Food Agric Enviro 2010; 8: 76-79.
- Yildirim AN, Akinci-Yildirim F, Polat M, Xan BS. Amygdalin content in kernels of several almond cultivars grown in Turkey. Hortscience 2014; 49: 268-1270.
- Gomez E, Burgos L, Soriano C, Marin J. Amygdalin content in the seeds of several apricot cultivars. J Sci Food Agric 1998; 77: 184-186.
- Hwang HJ, Kim P, Kim CJ, Lee HJ, Shim I. Antinociceptive effect of amygdalin isolated from Prunus armeniaca on formalin-induced pain in rats. Biol Pharm Bull 2008; 31: 1559-1564.
- Yildirim FA, Askin MA. Variability of amygdalin content in seeds of sweet and bitter apricot cultivars in Turkey. Afri J Biotechnol 2010; 9: 6522-6524.
- Krafft C, Cervellati C, Paetz C, Schneider B, Popp J. Distribution of amygdalin in apricot (Prunus armeniaca) seeds studied by Raman microscopic imaging. Appl Spectrosc 2012; 66: 644-649.
- Garcia MC, Gonzalez-Garcia E, Vasquez-Villanueva R, Marina ML. Apricot and other seed stones: amygdalin content and the potential to obtain antioxidant, angiotensin I converting enzyme inhibitor and hypocholesterolemic peptides. Food Funct 2016; 7: 4693.
- Yang C, Li X, Rong J. Amygdalin isolated from Semen Persicae (Tao Ren) extracts induces the expression of follistatin in HepG2 and C2C12 cell lines. Chin Med 2014; 9: 23.
- Ivan MS, Vesna DN, Ivana MS, Ljubisa BN, Mihajlo ZS. Development and validation of HPLC method for the determination of amygdalin in the plant extract of plum kernel. Res J Chem Enviro 2012; 16: 80-86.
- Song YQ, Zai DC, Chen L. Study on the amygdalin extraction in the kernel of loquat fruit. Resourc Develop Market 2006; 22: 210-212.
- Zhou C, Chen K, Sun C, Chen Q, Zhang W. Determination of oleanolic acid, ursolic acid and amygdalin in the flower of Eriobotrya japonica Lindl. by HPLC. Biomed Chromatogr 2007; 21: 755-761.
- Zhou CH, Li X, Zhang WS, Sun CD, Chen KS. Oleanolic and ursolic acid in the fruit of Eriobotrya japonica Lindl. J Med Plants Res 2011; 5: 1735-1740.