- Biomedical Research (2007) Volume 18, Issue 3
Detection of antibody and antigen in extrapulmonary tuberculosis patients' sera using a cocktail of mycobacterial excretory secretory antigens and their antibodies
Vijay Upadhye1, Niraj Shende1, Satish Kumar2and B C Harinath1*
1Jamnalal Bajaj Tropical Disease Research Centre, Mahatma Gandhi Institute of Medical Sciences, Sevagram, Wardha 442 102, India
2Department of Biochemistry, Mahatma Gandhi Institute of Medical Sciences, Sevagram, Wardha 442 102, India
- Corresponding Author:
- B. C. Harinath JB
Tropical Disease Research Centre & Department of Biochemistry
Mahatma Gandhi Institute of Medical Sciences Sevagram
442 102 (Wardha) M. S., India
Tele/ Fax: (07152) 284038
e-mail: jbtdrc_wda ( at ) sancharnet.in
Accepted date: April 11, 2007
Abstract
Increased incidence of extrapulmonary tuberculosis (EPTB) as co-infection is observed due to rise in human immunodeficiency virus (HIV) infection. However, the precise diagnosis of EPTB is faced with difficulty due to the lack of tissue biopsy or fine-needle aspiration cytology (FNAC) facilities in rural hospitals. Enzyme linked immunosorbent assay (ELISA) will be simple, convenient and doesn’t require sophisticated laboratory. Mycobacterial antigens ES-31, ES-43 and EST-6 antigens were isolated from Mycobacterium tuberculosis (M. tuberculosis) H37Ra bacilli by affinity chromatography. Cocktail of these antigens and their affinity purified antibodies were explored for detection of antibody and antigen by Indirect and Sandwich ELISA respectively. In a preliminary study with bacteriologically confirmed EPTB cases (n=32), assay of antibody/free antigen/immunecomplexed (IC) antigen showed a sensitivity of 100% and specificity of 90%. Based on this study, sera from suspected EPTB patients (n=164) diagnosed by clinical and other laboratory investigations, non-tubercular disease patients (n=75) and healthy controls (n=75) were screened. A sensitivity and specificity of 72% and 91% for antibody detection, 70% and 94% for circulating free antigen and 63% and 98% for circulating IC antigen detection were observed. On combining the positivity of antibody, circulating free and IC-antigen, overall sensitivity of 96% and specificity of 91% were observed in EPTB. Tuberculous antibody detection to cocktail antigen was found to be useful in detection of EPTB. However, circulating free and IC-antigen detection may be a better marker for detection of different groups of EPTB.
Keywords
Excretory secretory (ES) antigen, cocktail antigen, cocktail antibody, enzyme linked immunosorbent assay (ELISA), extrapulmonary tuberculosis (EPTB)
Abstract
Increased incidence of extrapulmonary tuberculosis (EPTB) as co-infection is observed due to rise in human immunodeficiency virus (HIV) infection. However, the precise diagnosis of EPTB is faced with difficulty due to the lack of tissue biopsy or fine-needle aspiration cytology (FNAC) facilities in rural hospitals. Enzyme linked immunosorbent assay (ELISA) will be simple, convenient and doesn’t require sophisticated laboratory. Mycobacterial antigens ES-31, ES-43 and EST-6 antigens were isolated from Mycobacterium tuberculosis (M. tuberculosis) H37Ra bacilli by affinity chromatography. Cocktail of these antigens and their affinity purified antibodies were explored for detection of antibody and antigen by Indirect and Sandwich ELISA respectively. In a preliminary study with bacteriologically confirmed EPTB cases (n=32), assay of antibody/free antigen/immunecomplexed (IC) antigen showed a sensitivity of 100% and specificity of 90%. Based on this study, sera from suspected EPTB patients (n=164) diagnosed by clinical and other laboratory investigations, non-tubercular disease patients (n=75) and healthy controls (n=75) were screened. A sensitivity and specificity of 72% and 91% for antibody detection, 70% and 94% for circulating free antigen and 63% and 98% for circulating IC antigen detection were observed. On combining the positivity of antibody, circulating free and IC-antigen, overall sensitivity of 96% and specificity of 91% were observed in EPTB. Tuberculous antibody detection to cocktail antigen was found to be useful in detection of EPTB. However, circulating free and IC-antigen detection may be a better marker for detection of different groups of EPTB.
Introduction
Early and precise diagnosis is essential to reduce morbidity and mortality in patients affected with tuberculosis (TB). Diagnosis of EPTB is often difficult to establish using standard methods. Moreover, because of difficulty in obtaining specimen from EPTB patients particularly in childhood and paucibacillary TB and delay in diagnosis due to time consuming and insensitive sputum and culture methods, immunodiagnosis seems to be an ideally suited diagnostic modality for EPTB. There is promise in serodiagnostic tests like ELISA because of their ease of performance and cost-effectiveness. Many groups have explored culture filtrate antigens of M. tuberculosis in ELISA for serodiagnosis of TB [1, 2]. M. tuberculosis excretory secretory (ES) antigens ES-31, ES- 41 and ES- 43 have been shown to be diagnostically useful in antibody detection [3-6]. Antigen EST-6 containing 38 and 41kDa proteins was also explored for antibody detection A cocktail of ES-31, ES-41 and ES-43 antigens had shown improved sensitivity compared to single ES-31 antigen in antibody detection in pulmonary TB [8]. Further, a cocktail of affinity purified antibodies against ES- 31, ES-43 and EST-6 antigens was explored for circulating free and IC antigen detection in TB by sandwich ELISA [9]. The present study explores the usefulness of cocktail of ES-31, ES-43 and EST-6 antigens and their specific affinity purified antibodies for detection of antibody and antigen respectively in EPTB by ELISA.
Materials and Methods
Patients and controls
Sera samples were received from 196 patients attending tertiary hospital, suspected of having EPTB based on clinical and other laboratory investigations. These patients belong to different forms of EPTB namely tuberculous lymphadenopathy (43), tuberculous meningitis (41), bone and joint TB (40), abdominal TB (39), pleural TB (13), genitourinary TB (8), ocular TB (8) and miliary TB (4). Out of these 196 patients, 32 cases were found to be AFB smear and/or culture positive respectively. These 32 bacteriologically confirmed EPTB cases includes tuberculous lymphadenopathy (8), tuberculous meningitis (5), bone and joint TB (6), abdominal TB (5), pleural TB (4) and miliary TB (4) cases respectively. This study includes 75 disease controls namely nonspecific lymphadenitis (n=13), pyogenic meningitis (n= 6), seizure (n= 5), encephalitis (n= 5), Rheumatoid arthritis (n= 12), ulcerative colitis (n= 12), Crohn’s disease (n= 4), Non-TB pleural effusion ((n= 5), ascitic (n= 3), Nephrotic syndrome (n= 2), infertility (n= 3), iridocyclitis (n= 1), abdominal abscess (n= 2) and chronic obstructive pulmonary disorder cases (n= 2). Sera samples from healthy individuals (n=75) with no history of TB were served as healthy controls. Sera samples were stored in 0.5 mL aliquots at - 20°C with 0.1% sodium azide until use. All cases included in this study had history of BCG vaccination. The study was done prospectively in blinded manner in which the clinical diagnosis was not available to the laboratory personnel. In the present evaluation studies, each sera sample has been assayed in triplicate.
Clinical history, physical examination, , baseline laboratory investigations (hemogram, tuberculin skin test, chest skiagram, glucose level in blood and Urinalysis), histological, cytological (FNAC), microbiological [acid fast bacilli (AFB) smear and culture] investigations, ultrasound or computerized tomographic (CT) scanning depending on the location of the infection in patients, clinicians’ high degree of suspicion of TB depending on clinical sign and symptoms or response to antitubercular therapy (ATT) were considered as the basis for confirmation of tuberculous aetiology. Most of the EPTB patients such as tuberculous lymphadenopathy (TBLN), bone and joint TB, abdominal TB, genitourinary TB and miliary TB cases were confirmed histologically, cytologically or by CT scan. The biochemistry and cytology of the cerebrospinal fluid and pleural fluid showing typical exudative nature with variable number of lymphocytic infiltration and raised protein level were also, taken into consideration for tuberculous meningitis (TBM) and pleural TB cases. In Ocular TB, all patients (n=4) presented with iridocyclitis were diagnosed as presumed ocular TB due to positive tuberculin skin test and cavitary lesions in chest skiagram.
Isolation of M. tuberculosis ES-31, ES-43 and EST-6 antigens and their antibodies
M. tuberculosis H37Ra detergent soluble sonicate (DSS) antigen, was prepared from M. tuberculosis H37Ra bacilli Briefly bacilli were 5% phenol inactivated in 0.5 M phosphate buffer saline (PBS, pH 7.2) and incubated with sodium dodecyl sulphate (SDS) extraction buffer. The supernatant was dialysed against 0.01 M PBS, pH 7.2 and used as an antigen source [9]. Goat anti-DSS IgG antibodies were raised in goat by immunizing intramuscularly with 500 μg protein / ml DSS antigen with 1 ml of Freund’s incomplete adjuvant on days 0, 20, 33 and 45. Immune sera were collected on days 32, 44, 57, 60 and thereafter fortnightly and anti-DSS IgG was isolated as described earlier [9]. M. tuberculosis ES-31 antigen was isolated from M. tuberculosis H37Ra DSS antigen by affinity chromatography using anti–ES-31 antibody-coupled Sepharose-4B column (Pharmacia Biotechnology AB, Uppsala, Sweden) [10]. Briefly, cyanogen bromide-activated Sepharose-4B beads were coupled with purified anti-ES-31 antibody. DSS antigen was passed through the column and ES-31 antigen was eluted by glycine–HCl buffer (0.01 mol/L, pH 2.5) and collected in Tris–HCl buffer (0.01M, pH 8.6). Similarly, ES-43 and EST-6 antigens were isolated separately from DSS antigen by affinity chromatography using anti-ES-43 or anti-EST-6 antigen– antibody coupled Sepharose-4B column. Cocktail antigen (ES-31, ES-43 and EST-6) was prepared by mixing the individual antigen in equal proportion. M. tuberculosis H37Ra anti–ES-31, anti–ES-43, and anti–EST-6 antibodies were isolated from anti–DSS IgG by affinity chromatography using ES-31, ES-43 or EST-6 antigen coupled Sepharose-4B column [10]. Cocktail antibody (anti–ES-31, anti–ES-43, and anti–EST-6) was prepared by mixing individual antibody in equal proportion.
ELISA
Indirect penicillinase ELISA was performed for detection of antibody using cocktail antigen. Optimum individual antigen concentration of 1 ng per stick was used in this assay. In brief, 5 μL of optimally diluted antigen (0.6-μg protein/mL) was applied to cellulose acetate membrane squares fixed to a plastic strip and used along with optimally diluted human serum (1:600). Serum incubation was done at 37°Cfor 1 h. After washings, strips were Screening of EPTB patients’ sera with cocktail of antigens and their antibodies 165 incubated with anti–human IgG penicillinase conjugate (1:1000) at 37°C for 30 min. Finally, strips were incubated with blue color starch–iodine–penicillin ‘V’ substrate. The sera samples showing complete decolorization of blue color of starch iodine penicillin ‘V’ substrate at least 5 min earlier than the negative control were considered as positive. Sandwich penicillinase ELISA was performed for detection of circulating free and IC antigen using cocktail of affinity-purified antibodies. Optimum individual antibody concentration of 1 μg protein per stick was used in the assay. In brief, 5 μL of optimally diluted antibody (0.6 mg protein/mL) was applied to cellulose acetate membrane squares fixed to a plastic strip and used along with optimally diluted human serum (1:300). Serum incubation was done at 37°Cfor 1 h. After washings, strips were incubated with goat anti–cocktail IgG penicillinase conjugate (1:1000) at 37°Cfor 1 h. Finally, strips were incubated with blue color starch–iodine–penicillin ‘V’ substrate. The sera samples showing complete decolorization of blue color of starch iodine penicillin ‘V’ substrate at least 5 min earlier than the negative control were considered as positive. For detecting IC antigen, serum samples were pretreated with glycine–HCl buffer (0.1 mol/L, pH 2.8) followed by heating at 65°Cfor 15 min as described earlier [11].
Results
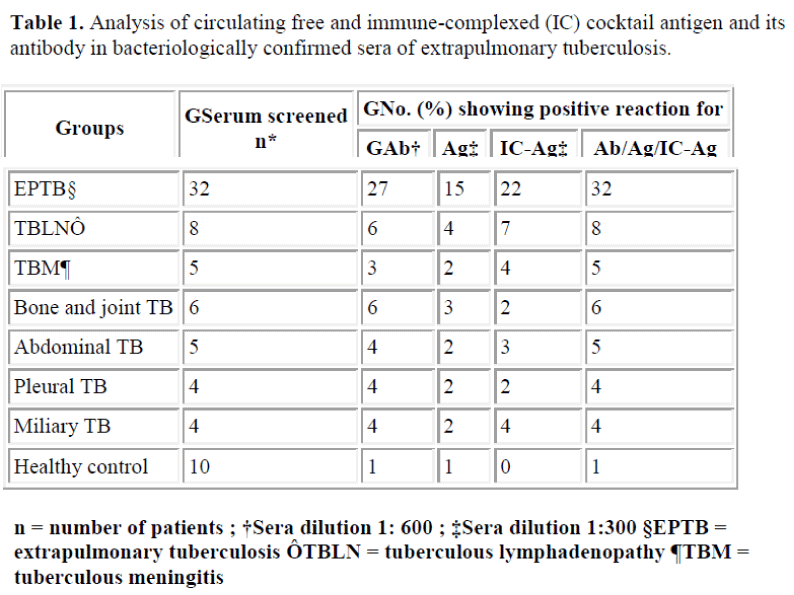
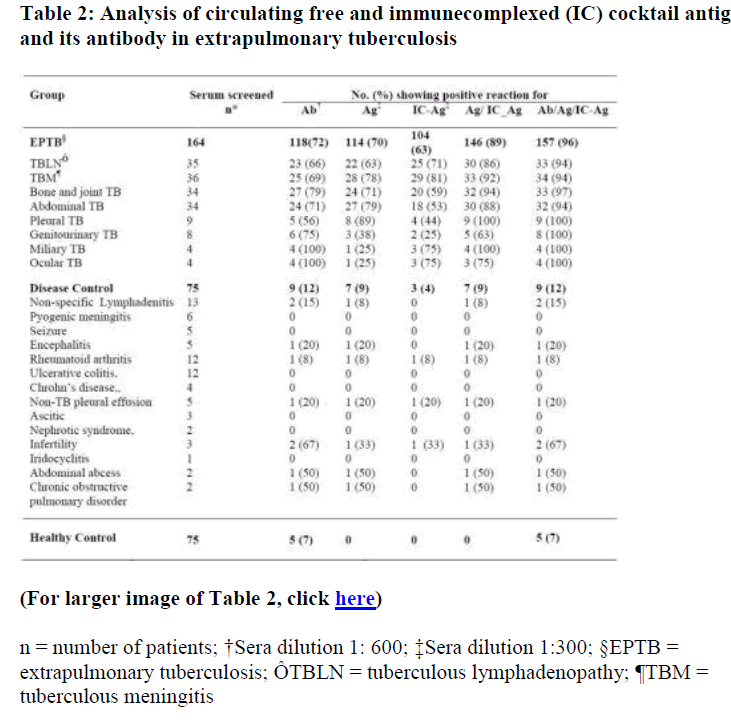
Bacteriologically confirmed EPTB patients (n=32) were analysed by antibody and antigen assay. Results are given in Table 1. Out of 32 bacteriologically confirmed EPTB sera screened, 27 (84%) cases showed presence of antibody, 15 (47%) for presence of free antigen and 22 (69%) for presence of IC antigen. A sensitivity of 100 % and specificity of 90% was observed for antibody/free antigen/ IC-antigen assay, showing usefulness of the assay system. Based on these observations, sera from 164 suspected EPTB patients diagnosed by clinical and other laboratory investigations, non-tubercular disease control (n=75) and healthy control (n=75) were screened for tuberculous IgG antibody using cocktail antigen and circulating antigen (free/IC-antigen) using affinity purified monospecific antibodies to the cocktail antigen (Table 2). Antibody positivity was observed in 118 (72%) cases of 164 suspected EPTB cases. Combination of free/ ICantigen assay has shown improved sensitivity of 89 % over sensitivity of 70% and 63% for individual antigen (free and IC antigen ) assay. On combining positivity for tuberculous antibody, free and IC-antigen (Ab/Ag/ICAg), sensitivity was increased to 96% compared to 72% for antibody detection. An overall specificity of 91% was observed for combined antibody and antigen assay.
(For larger image of Table 2, click here)
Discussion
EPTB constitutes about 15-20 % of all cases of TB while it accounts for more than 50% of cases in HIV-positive individuals [12]. Diagnosis of EPTB is often difficult because of different clinical presentations and nonavailability of sensitive or specific diagnostic tests. Many serological assays use single antigen (e.g. 38-kDa protein) that may not be recognized at all stages of infection [13]. Development of an assay based on the use of cocktail of specific antigens may help to solve this problem. Perkins et al observed increased sensitivity of antibody assay with the use of multiple antigens [14]. In TB infection, the antibody repertoire is highly diverse, with sera from different patients reacting with different antigens. Hence, immunodetection of circulating M. tuberculosis antigen secreted during active infection could be better diagnostic marker than antibody. Several studies have reported use fulness of detection of mycobacterial antigens such as plasma membrane antigen, antigen 5, lipoarabinomannan and cytoplasmic antigen [15-17]. Landowski et al reported higher level of Ag 85 in 97 Chilean TB patients by combinatorial use of antibodies [18]. Previous study showed usefulness of cocktail of affinity purified antibodies against M.tuberculosis ES-31, ES-43 and EST-6 antigens for detection of circulating free and IC-antigen in sera of confirmed TB patients by sandwich ELISA [9]. Based on the above studies, sera from hospital based suspected EPTB patients diagnosed by clinical and other laboratory investigations, were screened for detection of IgG antibody to M. tuberculosis cocktail antigen consisting of ESScreening of EPTB patients’ sera with cocktail of antigens and their antibodies 165 31, ES-43 and EST-6 antigens by indirect ELISA. There are very few published reports available on the use of serology in abdominal, bone and joint and pleural TB. Of the available reports, Demkow et al [19] observed 56 % sensitivity and 99 % specificity using 38 kDa and 16 kDa antigen in bone and joint TB cases for detection of tuberculous antibody. In our study, cocktail antigen was observed to be more reactive in bone and joint TB with 79% sensitivity and 92% specificity for IgG antibody detection. Data on the serodiagnosis of genitourinary TB, miliary TB and ocular TB by ELISA are very scarce. In the present study, detection of antibody to cocktail antigen in genitourinary TB (6/8), miliary TB (4/4) and in clinically presumed ocular TB (4/4) cases was observed to be useful for diagnosis of TB. The presence of immune complexes in patients with active pulmonary TB and their diagnostic significance have been emphasized in earlier reports [20- 23]. Hence in the present study, the use of cocktail antibody consisting of specific anti- ES-31, anti-ES-43 and anti-EST-6 antibodies was explored in sandwich ELISA for circulating free as well as IC-antigen detection. The study showed that circulating IC-antigen is a sensitive marker than circulating free antigen in TBLN (71%) and TBM (81%) cases. Earlier, Dhand et al [24] has reported false positivity in the serological diagnosis of pleural effusion.
But with the use of cocktail antibody in Pleural TB, we could observe 8 cases positive out of 9 for free antigen detection. Cocktail antibody was also found to be sensitive for free antigen detection in abdominal TB (79%). Detection of antibody/free antigen/IC-antigen in EPTB patients showed an overall sensitivity of 96%. Of these 164 cases when followed, 44 patients were started on ATT after ELISA test. This includes TB patients with TBLN (10), TBM (9), bone and joint TB (11), abdomen TB (2), pleural TB (3), genitourinary TB (1), ocular TB (4) and miliary TB (4). Out of 75 disease control sera, 9 cases (12%) showed positivity similar to earlier reports with ES-31, ES-41 and ES-43 antigen used individually or in combined form [3-6,8,9]. The observed crossreactivity of control sera may possibly due to the exposure to environmental mycobacteria. These cases need to be followed for development of TB disease if any. The overall specificity for combined antibody and antigen assay was found to be 91 %. In the present study using cocktail of antigens and their antibodies, has shown good diagnostic potential and thus useful in confirming EPTB along with other clinical and laboratory findings. Screening of sera for tuberculous IgG antibody to cocktail antigen was found to be useful in detection of EPTB. However, circulating free and IC antigen detection may be a better marker for diagnosis of different groups of EPTB.
Acknowledgement
This study was supported by a research grant from Kasturba Health Society and CSIR. Thanks are due to Shri. Dhiru S. Mehta, President, KHS and Dr. (Mrs.) P. Narang, Dean, MGIMS for keen interest and enouragement, Dr. Kalsait, district tuberculosis officer and staff, Civil Hospital, Wardha, for cooperation and Mrs. S. Ingole for technical assistance.
References
- Nassau E, Parson ER, Johnson GD. The detection of antibodies to Mycobacterium tuberculosis by enzyme linked immunosorbent assay (ELISA). Tubercle 1976; 57: 67-70.
- Benjamin RG, Debanne SM, Ma Y, Daniel TB. Evaluation of mycobacterial antigens in an enzyme linked immunosorbent assay (ELISA) for serodiagnosis of TB. J Med Microbiol 1984; 18: 309-318.
- Banerjee S, Gupta S, Kumar S, Shrikhande AV, Reddy MVR, Harinath BC. Seroreactivity of 31 kDa and 41 kDa mycobacterial secretory proteins isolated from culture filtrate in extra pulmonary tuberculosis. Indian J Pathol Microbiol 2003; 46: 261-264.
- Gupta S, Shende N, Kumar S, Harinath BC. Antibody response to M.tb.H37Ra excretory-secretory ES-43 and ES-31 antigens at different stages of pulmonary tuberculosis. Biomed Res 2004; 15: 76-79.
- Banerjee S, Gupta S, Shende N, Kumar S, Harinath BC. Serodiagnosis of tuberculosis using two ELISA systems. Ind J Clin Biochem 2003; 18: 48-53.
- Bhatia AS, Gupta S, Shende N, Kumar S, Harinath BC. Serodiagnosis of childhood tuberculosis by ELISA. Ind J Pediatr 2005; 72: 383-387.
- Lodam AN, Reddy MVR, Narang P, Gupta OP, Harinath BC. Fractionation, analysis and diagnostic utility of Mycobacterium tuberculosis H37Ra excretorysecretory antigen in pulmonary tuberculosis. Indian J Biochem Biophys 1996; 33: 66-71.
- Gupta S, Shende N, Kumar S, Harinath BC. Detection of antibodies to a cocktail of mycobacterial excretorysecretory antigens in tuberculosis by ELISA and Immunoblotting. Curr Sci 2005; 88: 1825-1827.
- Harinath BC, Kumar S , Saha-Roy S , Hirudkar S, Upadhye V, Shende N. A cocktail of affinity-purified antibodies reactive with diagnostically useful mycobacterial antigens ES-31, ES-43 and EST-6 for detecting the presence of Mycobacterium tuberculosis. Diagn Microbiol Infect Dis 2006; 55: 65-69.
- Nair ER, Banerjee S, Kumar S, Reddy MVR, Harinath BC. Isolation of Mycobacterium tuberculosis 31 kDa antigen protein of diagnostic interest from culture filtrate using anti ES-31 antibody by affinity chromatography. Indian J Clin Biochem 2001; 16: 132-135.
- Prasad GBKS, Kharat I, Harinath BC. Detection of antimicrofilarial ES antigen- antibody in immune complexes in bancroftian filariasis by enzyme immunoassay. Trans R Soc Trop Med Hyg 1983; 77: 771-772.
- Sharma SK, Mohan A. Multidrug resistant extrapulmonary tuberculosis in a HIV-negative patient. Indian J Tuberc 2004; 51: 43- 46.
- Pai M, Kalantri S, Dheda K. New tools and emerging technologies for the diagnosis of tuberculosis: Part II. Active tuberculosis and drug resistance. Expert Rev Mol Diagn 2006; 6: 423-432.
- Perkins MD, Conde MB, Martins M, Kritski AL. Serologic diagnosis of tuberculosis using simple commercial multiantigen assay. Chest 2003; 123: 107-112.
- Krombovitis E, McMurry MB, Lock PE, Hendrickse W, Holzel H. Rapid diagnosis of tuberculous meningitis by latex particle agglutination. Lancet 1984; 2: 1229-1231.
- Radhakrishnan VV, Mathai A. Enzyme linked immunosorbent assay to detect M. tuberculosis antigen 5 and antimycobacterial antibody in cerebrospinal fluid of patients with tuberculous meningitis. J Clin Lab Anal 1991; 5: 233-237.
- Kadival GV, Mazarelo TBMS, Chaparas SD. Sensitivity and specificity of enzyme-linked immunosorbent assay in detection of antigen in tuberculous meningitis. J Med Microbiol 1985; 20: 901-904.
- Landowiski CP, Godfrey HP, Bentley-Hibbert SI, Liu X, Huang Z, Sepulveda R, Huygen K, Gennaro ML, Moy FH, Lesley SA, Haak-Frendscho M. Combinatorial use of antibodies to secreted mycobacterial proteins in a host immune system-independent test for tuberculosis. J Clin Microbiol 2001; 2418-2424.
- Demkow U, Zielonka TM, Nowak-Misiak M, Filewska M, Bialas B, Strzalkowski J, Rapala K, Zwolska Z, Skopinska-Rozewska E. Humoral immune response against 38 kDa and 16 kDa mycobacterial antigens in bone and joint tuberculosis. Int J Tuberc Lung Dis 2002; 6: 1023-1028.
- Brostof J, Lenzini L, Rottali P, Rottalli L. Immune complexes in the spectrum of tuberculosis. Tubercle 1981; 62: 169-173.
- Johnson NM, McNicol MW, Burtan-Kee EJ, Mowbray JF. Circulating immune complexes in tuberculosis. Thorax 1981; 36: 610-617.
- Harrington JJ III, Ho J L, Lapa e Silva JR, Conde, MB, Kritski AL, Fonseca LS, Saad MH. Mycobacterium tuberculosis lipid antigens: use of multi-antigen based enzyme immunoassay for free and complex dissociated antibodies. Int J Tuberc Lung Dis 2004; 4: 161-167.
- May JJ, Katilus J, Henson PM, Dreisin RB. The purification and identification of circulating immune complexes in tuberculosis. Am Rev Respir Dis 1983; 128: 922-925.
- Dhand R, Ganguli NK, Vaishnavi C, Gilhatra R, Malik SK. False positive assay of Mycobacterium tuberculosis antigens in pleural fluid. Med Microbiol 1998; 26: 241-243.