Research Article - Allied Journal of Medical Research (2020) Volume 4, Issue 2
Detection and evaluation of ELISA analysis for the circulating cancers sera antigens by monoclonal antibody UNIVmAB and pembrolizumAB.
D Manjunath1, Vishal V Immanuel1, Naveen Puttaswamy1, Sashidhara AV2, Anil Thomas1 and Shib D Banerjee3*
1Preethi Center of Oncology, Vattavyalil Cancer Trust, Mysore, Karnataka, India
2Logic and Clue Diagnostics, Mysore, India
3Department of Anatomy and Cellular Biology, Tuft University School of Medicine, Boston, MA, USA
- Corresponding Author:
- Shib D Banerjee
Department of Anatomy and Cellular Biology
Tuft University School of Medicine
Boston, MA USA
E-mail: sdbaner@yahoo.com
Accepted date: April 7, 2020
Citation: Manjunath D, Immanuel VV, Puttaswamy N, et al. Detection and evaluation of ELISA analysis for the circulating cancers sera antigens by monoclonal antibody UNIVmAB and pembrolizumAB. Allied J Med Res 2020;4(1):45-52.
Abstract
Detection and diagnosis of multiple human blood cancers sera remain a challenge, Recently we have reported a research note on a common sera biomarker for various cancers sera. However, a highly sensitive assay is required for the early and late cancers detections. We have developed an ELISA for an antigen detected by a specific monoclonal antibody, named UNIVmAb and with pembrolizumAb for comparison in all human cancers of different grades.
This first method is based on the UNIVmAb antibody reaction, which is highly accurate and reproducible for serum antigen present in sera of different grades of cancer patients compared to the normal subjects. In this report, human sera from >90 cancer patients with known TNM classification along with sera from 72 normal healthy individuals were analyzed by UNIVmAb/PembrolizumAb for blood cancers sera antigen. This ELISA using UNIVmAb has identified the antigen from human sera as a complex hyaluronan binding protein composed mainly of multiple unknown proteins, including human serum albumin. The limit of detection for the antigen ELISA with UNIVmAb is 4ng to 12ng, giing>80%-85% higher values than the healthy donors. However, anti PD 1(PembrolizumAb) is 30%-40% higher the healthy donors in most of the cancer patients. We validate enzyme-linked immunosorbent assay (ELISA) for quantification of human sera specific antigen in various cancers sera samples and to compare the protein levels in normal subjects to cancer patients. Such results indicate that this ELISA value may be useful as a diagnostic marker for various cancer patients with UNIVmAb than with anti PD 1 antibody. Even though the use of immune checkpoint inhibitors have provided a breakthrough in immunotherapy for malignant tumors, PembrolizumAb or Nivolumab has significantly improved the survival of patients with melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma, and classic Hodgkin lymphoma . Biomarkers that predict the treatment response to immune checkpoint inhibitors have also been explored. PD-1 expression in tumor cells was associated with objective response rates (ORRs) to nivolumab in some studies.
Clinically approved immune checkpoint inhibitors such as anti-PD1 or anti-CTLA 4 demonstrate clinical efficacy in only a small proportion of cancer patients. The majority of cancer patients either show primary resistance or develop acquired resistance. The present data with ELISA indicate that the UNIVmAb is more applicable in detecting all primary or progressive cancers in circulating human sera and potentially can be used as a common cancer biomarker.
Keywords
ELISA, Biomakers, Monoclonal antibody,Immunoglobulins.
Abbreviation
ELISA: Enzyme-Linked Immunoassay;UnivmAb: Monoclonal Antibody;anti PD1: PembrolizumAb; H11: Serum Antigen; HABP: Hyaluronan Binding Protein;HA: Hyaluronan; PBST: Phosphate Buffer Triton.
Introduction
The cancer diagnosis is defined as a molecular expression of cancer-associated proteins by analyzing the specific protein expression patterns of tumor cells or extracellular fluids such as sera from cancer patients. To date, there have been many sera proteins suggested for various types of cancer. However, none has yet met the stringent requirements for clinical use, such as reproducibility, specificity, sensitivity too many cancers [1-10].
Before cancer prevention becomes a reality, early detection and better treatment remain the hope for all cancer patients. It is easy to understand that the test for early detection of cancer is best done in sera or other types of body fluids. On the other hand, cancer classification and management may be accomplished by using biopsy tissues, between different types and progressive cancers, The problem of detection of cancers in sera is impeded by the presence of highly abundant proteins such as albumin, immunoglobulins, transferrin, haptoglobin, lipoprotein and, antitrypsin which constitute more than 85% of the serum proteome. Cancer proteins are secreted from cells of metastatic origin into the circulatory system. Intensive investigations of common cancers sera biomarker have been reported [1]. However, the identification of a single protein for several different cancers has eluded identification, such as CA-125, serological mucin assay for pancreatic cancer, serum cytokine IL-7, EGF, VEGF, monocyte chemotactic protein (MCP 1) IL6 and 8 in ovarian cancer, serum GFAP (glial fibrillary acidic protein) in glioblastoma multiform, serum CEA in colorectal cancer. There are multiple proteins for pancreatic adenocarcinoma such as C-reactive protein, CA 242, haptoglobin, M2-pyruvate kinase, IGF binding proteins, etc, however, CA-19-9 is used most extensively [2-18]. Also evaluating methylation of cell-free DNA studies within a blood sample may detect various GI cancers have proven clinically useful, often in combination with other diagnostic tools [10]. The assay was developed based on findings from the multicenter, prospective Circulating Cell-Free Genome Atlas (CCGA) study. This noninvasive blood testing is still advancing, in terms of both the depth of sequencing that can be done and the cost of testing. However, Some of the cancers are also detected by the anti-PD-1 antibody.One molecule that is present in multiple tumors is hyaluronan [18,19]. Hyaluronan interacts with several various cell surface receptors, including aggrecan, link protein, versican, neurocan CD44, RHAMM, LYVE-1, HARE/stabilising-2, Toll-like receptors 2 and 4 (18,19,22-24). TSG6 , GHAP, and H11 [1,11,21-28]. The interaction between the HA-Hyaluronan receptor plays a significant role in tumor progression [11,27].
Hyaluronan (HA) composition is well defined and it is a ubiquitous component of the extracellular, pericellular, and, intracellular compartments of tissues as well as present within the circulation. It is known to mediate cell proliferation, migration, and adhesion and to regulate key events in development, inflammation, and tumor. The interaction of HA with specific cell surface receptors known as hyaladherinsregulates cell phenotype [18-20].
Most malignant tumors such as colon carcinoma, breast carcinoma, gliomas, lung carcinomas, mouse mammary carcinoma, melanoma cells, and Wilms' tumors contain elevated levels of HA than their normal counterparts [17]. However, the increase in hyaluronan binding protein or proteins is evident in the sera.
Immunotherapeutic research can lead to the identification of human sera antigen and help to the diagnosis of early cancer. The number of studies on the validation of ELISA from human sera from breast cancer patients tyrosine kinase [29], human aloha-1 antitrypsin, quantification of human collectin11, human saliva samples, validation of Herceptin, blood serum proteins such as OPN, VEGF in non-small lung cancer, detection of rituximab in serum and increase serum level of Hepcidine and GDF-15 linked to metastasis in Renal cell carcinoma and Urothelial carcinoma is reported. We have identified a monoclonal antibody (clones H11B2C2) to the tumor-associated protein and was reported as a tumor biomarker. The same Mab may be used for various cancers sera profile. However, accumulating evidence suggests that only a fraction of cancer patients benefit from checkpoint inhibitors (pembrolizumAb/ipilimumAb), and severe immunerelated adverse events (irAEs) are seen in some patients undergoing immune checkpoint inhibitor therapy [29-37].
Analyses of peripheral blood/circulating cancers sera are noninvasive methods with good potential to predict treatment outcomes even after immune therapies. Also, reports have shown that in various malignancies, increased tumorinfiltrating immune cells and peripheral blood absolute lymphocyte count (ALC) can be utilized as predictive biomarkers [7-9].
Therefore, the development of predictive biomarker is critical for patients coming to the clinic to avoid any adverse effects. For a sensitive assay, we have adapted the ELISA technique because ELISA uses the immunological concept of an antigenbinding to a specific antibody which helps to detect minimal antigen present in the sera. In this study, we have named the original clones H11B2C2 producing antibodies as UNIVmAb for the main reason that all progressive tumors showed overexpression of the same tumor antigen. This method is based on the UNIVmAb antibody reaction, which is highly accurate and reproducible for the cancer antigen present in sera of different grades of cancer patients compared to the normal subjects [11].
We have identified the antigen from human cancer sera as a complex Hyaladherin, composed mainly with multiple unknown proteins including IgHGI, IgG superfamily and, are associated with human serum albumin. Immunoglobulin superfamily, IgHGI and, a minor component HSPG (Papers in Preparation). The limit of detection for the antigen by ELISA is 4ng to 12ng, giving >80-85% higher values than the healthy donors. This value also indicates the best diagnostic marker for cancer sera for various cancer patients. Thus the objectives of this present work are to develop and validate enzyme-linked immunosorbent assay (ELISA) for detection and quantification of human sera specific antigen in eighteen different cancers sera samples and to compare protein levels in normal subjects to cancer patients both with UNIVmAb and with PembrolizumAb and developed a predictive reliable diagnostic biomarker.
Materials and Methods
Sample collection and preparation
72 normal subjects and >90 cancer patients of various stages were studied. Serum samples from normal subjects and cancer patients accessed from cancer hospitals in Mysore, India (Preethi Centre for oncology, KR Hospital, Logic and Clue diagnostic) and the protocol approved by the ethical review committee and the patient's consent were taken. Blood samples were collected from each patient before any treatment, e.g. radiation or chemotherapy. Samples centrifuged at 2000xg for 30 min after standing at room temperature for 1 h, and the separated serum was stored either at -800°C or in liquid nitrogen. The tumor sections from patients after HandE stain were graded using the TNM grading system. Serum samples treated with 4×lysis buffer, containing 0.2 M Tris-HCl (pH 8.0), 80 mM EDTA, 4 mM PMSF, 4 mMBenzamidine-HCl and 2% Triton × 100 plus protease inhibitor cocktails (Sigma) were centrifuged at 10,000xg for 30 min at 40°C. The supernatant was stored either at -800C or in liquid nitrogen until further analysis for western blot work. The amount of protein was estimated at UV 280 nm and Bradford reagent using Bovine serum albumin as a standard. (Sigma Protocol,Sigma). The anti-PD1 antibody, Pembrolizumab purchased from (cat# 329902. Biolegend, San Diego, U.S.A).
Production of monoclonal antibody UNIVMab
Hybridoma and the antibody were prepared, according to Boregowda [11,25,27]. In brief, the hybridoma was grown in DMEM under pathogen and complement free human serum received from the hospitals or in serum-free media from Gibco. The antibody production in human serum of any blood groups did not affect UNIVmAb in recognizing the human antigen expressed in tissues and serums derived from various malignant tumors. The clones were grown in DMEM, containing 10% (v/v) inactivated human serum. After 21 days, the media were collected and precipitated with cold saturated ammonium sulfate solution (final 50%) with constant stirring at 40C overnight and centrifuged at 12000xg for 30 min. The pellet was dissolved in PBS and dialyzed against PBS. The antibody solution lyophilized and antibody dissolved in PBS whenever required.Similarly, hybridoma was grown in serumfree media (SFM) was subjected to the same preparation as above for the antibody production.
Validation of UNIVMab antibody for the detection of serum antigen (H11)
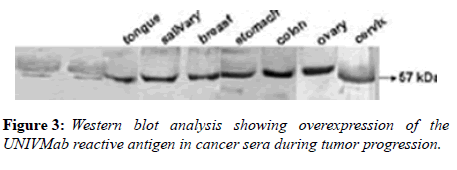
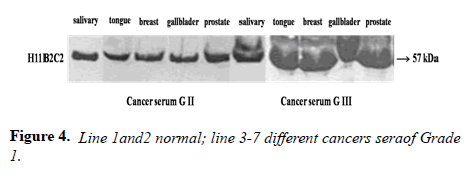
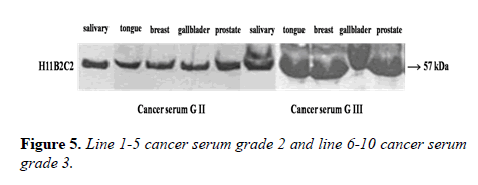
Parameters tested during assay validation were precision, accuracy, lower limit and upper limit quantification, selectivity, stability, and range. All samples were tested in triplicate. Reproducibility was evaluated through independent measurement of control sera and cancer sera providing various concentrations of the UNIVmAb antibody and its reaction to the antigen. The antibody showed higher expression both by western blot and immune-precipitation with two distinct molecular proteins at 57 and 32kd specifically in all cancers samples.
Western blot analysis of serum
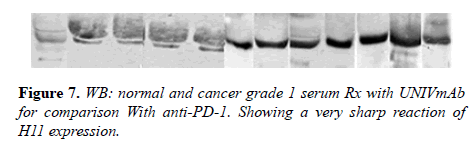
15 μg proteins from serum lysate were resolved on 10% SDS-PAGE under reducing condition and transferred to the PVDF membrane. The membrane was blocked with 5% skimmed milk in TTBS for 1h. After blocking, the blots were incubated mAb (1:1000 dilution) overnight at 4 0C. Following day, UNIVMab/Pembroizumab reacted blots were incubated with biotinylated goat anti-mouse IgG (1:10000 dilution) for 1h at room temperature and washed with TTBS. Reacted blots incubated with a 1:15000 dilution of streptavidin-peroxidase (HP09) for 1h at room temperature, and the specific proteins detected using Enhanced Chemiluminescence (ECL). Alternatively, after the membrane reacted with the primary antibody for overnight reacted directly with HRP conjugated goat anti-human antibody at 1:10000 dilution in TTBS for 1 hour at R.T. and processed as above.
ELISA assay with UNIVMab/PembrolizumAb for the detection of sera protein
MaxiSorp flat-bottom of high protein binding capacity polystyrene-96 well plates were used. Plates are coated by incubation with various concentrations (4ng to 12ng) of sera protein without lysis buffer from various cancer patients and normal subjects in a coupling buffer of pH 9.6 (Carbonate bicarbonate) overnight at 4˚C. Plates are washed with PBS three times and blocked with 100ul skimmed milk in PBS for 1 hour. Wash plate 3× with PBS-Tween. Primary antibody (UNIVMab/anti-PD 1) diluted in PBST 1:10,000 is added to the plates and incubated overnight at 4˚C. Biotinylated goat anti-mouse IgG at 1:20,000 dilutions in PBST is added and incubated at room temperature for 1 hour. Wash plates 3× with 100μl PBST. Streptavidin peroxidase is diluted in PBST at 1:50,000 dilutions and incubated for 1 hour at room temperature. Wash plates 3x with PBST. The reaction is developed with 100μl of mg/ml ABTS in 0.1M citrate buffer at pH 4.0 and 5% hydrogen peroxide. Developed for 30 minutes and the reaction is stopped with 100μl of 0.2M citric acid and read at 405nm. Control BSA at various concentrations ran at the same time along with the experimental samples.
Each experiment calibrated with the standard amounts of known proteins, and a linear concentration-dependent curve was established before the measurement of samples. Each sample used in triplicate and the experiments were repeated at least three times. Protein levels by quantitative ELISA. The working range was based on combinatory evaluation of the coefficient of variation (CV), the measured/mean ratio and, the linearity of the dilution curves for serum from serum donors. CV was acceptable (<10%) in the range 2.0 ng/ml-12.0 ng/ml and the measured/mean ratio was acceptable (<20% deviation from mean) in the range 4 ng/ml–12.0 ng/ml. The linearity of diluted samples was found acceptable (<20% deviation from the mean) in the range. Based on these findings, the working range of the ELISA was determined to be 4–12 ng/ml. The lower detection level was 2ng/ml.
Results and Discussion
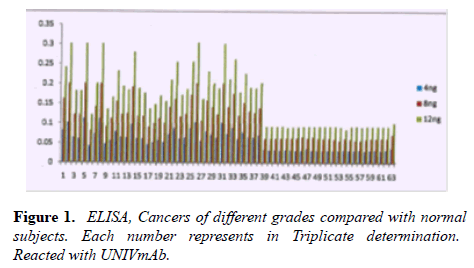
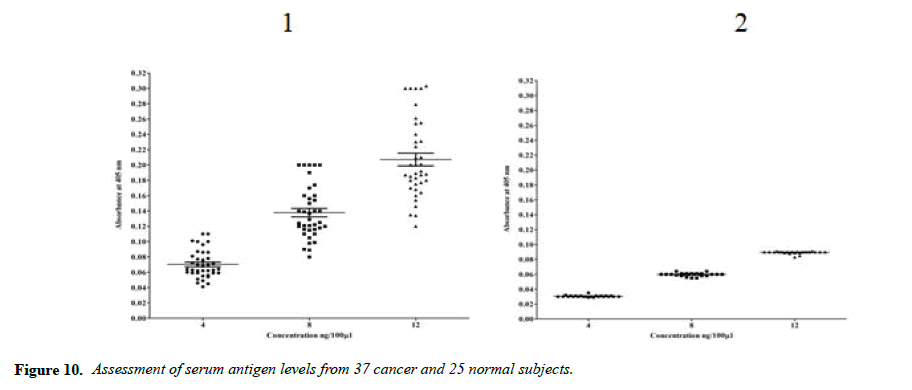
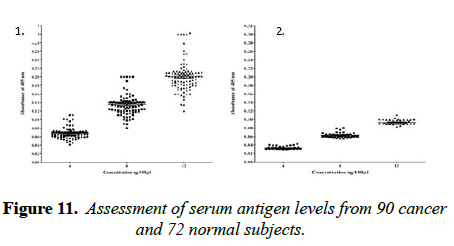
The main objective of this study is to detect sera cancer antigen using UNIVmAb and compared with anti-PD1 antibody, PembrolizumAb-PD-1–targeted therapies have shown clinical activity in a wide variety of cancer types. However, not all patients experience clinical benefits from PD-1 therapy, and there is a critical need to determine the requirements for improving current therapies but also to identify predictive biomarkers. We are eagerly searching for common biomarkers molecular targets which are usually extracellular, either on the surface of cells or circulating in the blood/sera or other tissues. Screening hybridomas and other libraries for antibody binding to each of these types of targets have historically involved the use of enzyme-linked immunosorbent assays (ELISA) that would tell them if immunotherapy-which can take time to show the definitive result is working in a patient. However, advancement in this field is dependent on the successful discovery and development of novel therapeutic antibody candidates, perform multiplexed screens for antibody binding to either cell surface or to circulating target antigens. In our work, the TNM classification was compared with the outcome of the cancer grading as we detected the precision of ELISA was determined to assay every sample in triplicate and assaying four times on one plate. The average inter-assay variation was less than 5% in control samples, whereas the average of inter-assay variation was ̴ 10%. The sensitivity of the assay was between 4ng to 12ng was the concentration that gave an absorbance of the zero standards plus three standard deviations. The level of sera protein, antigen (named H11) detected by UNIVmAb was measured in more than 90 × 3 cancer patients. ELISA yielded much higher value for the protein (serum antigen) H11 in cancer than the normal subjects. Also, the advantage of this sensitive ELISA assay for the different grades of cancer gave a very reliable determination of the expression of the protein in sera during cancer progression. Figure 1 represents a comparison between grades one, two and grade three sera and the normal showing a definite significant increase in the activity of serum antigen (H11) expression average up to 50-95% higher level depending on the grade of cancer than the normal sera. For example, Figure 1 showed results from 37 cancers and 25 normal patients even though we have analyzed in more than 90 cancer patients and >72 normal subjects. Similar results were observed whether it was from cancer grade 1,2 or 3, and the normal subjects showed less than 60 to 75% average of the cancer activity depending on the grade with UNIVmAb while reacting with anti-PD1 normal subjects showed 15 to 25% less of the high- grade cancer sera.
Note: 1.ovary-III, 2.stomach-I, 3.blood cancer-III, 4.liver and lung-II, 5.tongue-II 6.ovary-II, 7.alveolai-III, 8.stomach-III, 9.stomach-I, 10.ovary-III, 11.-II, 12.base of tongue-II, 13.breast-III, 14.ovary-II, 15.tongue 16.thyroid, 17-II, 18.nostril, 19.ovary-II, 20.oseophagus-III, 21.-II, 22.rectum-II, 23.stomach-I, 24.breast-III, 25.base of the tongue – II, 26.breast-II, 27.oseophagus-III, 28.breast-II, 29.breast-II, 30.sarcoma –II, 31.NHL-III, 32.lymphoma-II, 33.The base of tongue-I, 34.pancrease-IV, 35.endometrium-I,36.endometrium-II, 37.endometrium-III. 38 to 63 Control human sera.
Note: 1.Gr2 2:.gr2.,3: gr3., 4: gr4., 5: gr3 6: gr3.,7:gr 3., 8: gr2.,9: gr3.,10: gr4.,11:gr211: gr3., 12:gr2.,13:gr3.,14: gr 2., 15:grgr3.,16:gr3.,17to22:gr Normal. Green for12ng, Red for 8ng and Blue for 4ng of serum proteins.
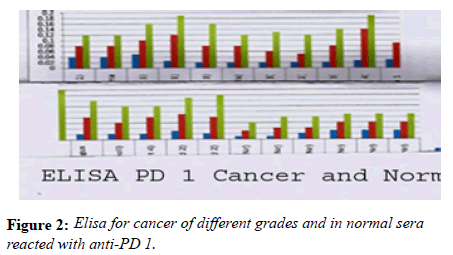
Many studies have shown the importance of this antigen detected by UNIVmAb not only during tumor progression but also in human cancer sera of different grades [1,11,27]. To validate the ELISA data both with UNIVmAB and PembrolizumAb we presented western blot data and investigated the specificity of the antibody with western blotting of various cancers and normal sera that followed by HA-oligo competition Western blot results clearly showed the presence of overexpression of cancer serum antigen in grade 2 and 3 compared to normal sera especially with UNIVmAb. However, HA-oligo (8-16 HA-oligomers, 150ug) competition showed more than 65% (image analysis) reduction of the expression of the antigen both in UNIVmAb and anti-PD1 (Figures 2-9).
It is known that HA-Oligo can compete for endogenous polymeric hyaluronan, high-affinity multivalent receptor interaction with low affinity, low valency interaction to be a member of hyaladherin [28]. When we compared the western blot data between UNIVmAb and PD1 antibody reaction we find significant differences in antigen expression. Image analysis showed at; east 40 to 50% overexpressed in UNIVmAb reaction than with the anti-PD1. Besides, the anti- PD1 reaction didn’t show many differences in expression in progressive cancer sera. However, it was clearly showed continued overexpression of H11 antigen from grade two to grade three cancer sera with UNIVmAb (Figures 2-9). This result may indicate the UNIVmAb is more sensitive in the increase in cancer antigen expression during progression (Figures 10 and 11). Cancer serum antigen concentration was measured from >90 cancers and 72 normal patients. A, B showed the assessment of serum antigen expression by Graph Pod Prism Version 5 software. The mean serum antigen level in normal subjects is 2ng/ml with 90% confidence of 2-0.1ng/ml. Normal serum antigen below the working level of the assay. While in cancers, patients showed a value of 0.02 to 0.06ng. However, the sera antigen showed both by Western blot and ELISA other protein or proteins present in addition to Hyaluronan. To determine whether this antigen is an albumin carrier protein. We assess the overexpression of cancer serum antigen is a species of albumin associated binding proteins by using Cibacron blue gel exclusion method. Eluted material with acetonitrile, water, trifluoroacetic acid and reacted with bhyaluronan and UNIVMab. Both b-HA and UNIVmAb reacted at the same molecule. Wash material at 57kD named H11 as cancers sera antigen. We found the serum antigen even though we named H11 as we determined a proteomic analysis of 57kD protein from colon samples conducted by Karolinska Institute of Sweden, showing mainly IgGH1, an immunoglobulin superfamily (Paper in preparation). Currently, Monoclonal antibodies are being used as potential therapeutics against cancer. Following the development of hybridoma technology by Köhler, combined with serological techniques and analytical tools, monoclonal antibodies (mAbs) were used to analyze the surface structure of human cancer cells and as a possible biomarker in cancer sera, thus paving the way for the identification and quantification of cancer-specific antigens suitable for targeting by antibodies. Found a common binding protein (HABP) that is ubiquitously overexpressed at markedly higher levels in various types of the malignant tumor compared with benign using clones H11B2C2 mab (UNIVMab) [11]. Thus the overexpression of UNIVmAb-reactive H11 can be used as a potential biomarker during tumor progression [1,11,27,28]. Unfortunately, a high concentration of human serum was used during western blot work which confirmed the validity of UNIVmAb or PembrolizumAb reactive antigen even though it is contrary to ELISA where minimal serum protein can be used to get viable and sensitive assay with UNIVmAb antibody. Also, one can use 1:10000 dilution of 0.5μg/ml antibody. It's economical and highly specific and accurate in determining the reactivity. In ELISA work, it is always advisable to avoid higher value both for sera protein and antibody, which I believe is inherent to the ELISA methodology. It is better to reiterate that the current ELISA was thoroughly validated, reliable and specific. Evaluation of mAb reactive cancers sera antigen from 21 different cancers sera types of various grades is reported earlier in Table form (BMC research note 2019). ELISA is unaffected by the presence of other diseases except in cancer cases. The overexpression of UNIVmAb or anti-PD1 reactive serum antigen by ELISA also can be used not only as a potential diagnostic marker during tumor progression but also for early detection of cancers. Furthermore using the UNIVmAb antibody in the ELISA method is the more sensitive and accurate tool to measure the cancer protein level from human cancers sera. Our data generate a new tool to study a specific cancers sera protein level and eventually provide a diagnostic strategy. The questions naturally are raised whether this mAb can be used as a potent therapeutic application for all progressive human tumors. Because the Hybridoma that produces the mAbs is capable to maintain an extremely high affinity towards their target of various cancers of different grades as observed in our ELISA work, we assumed the hybridoma cells may provide the answer (Paper in preparation) for therapeutic intervention. Due to this high affinity and specificity, we began investigating the therapeutic potential of Hybridoma-mAbs as metabolic activators, inhibitors and immuno-modulators. Preliminary results showing the elimination of circulatory cancer sera antigen from various cancers sera investigated.
Note: Cancer serum of different concentration 4, 8 and 12ng per 100 microlitres. The mean values are 0.07, 0. 13 and 0.21 respectively.
Note: Normal serum of different concentration 4, 8 and 12ng per 100 microliters. The mean values are 0.03, 0.06 and 0.09 respectively.Mean values of serum levels are indicated with a solid line.
Conclusion
Finding a common cancer Biomarker even though for several decades was a challenge, we believe our Hybridoma which produces the UNIVmAb now can meet that challenge after screening twenty different cancers sera of different grades after comparing with PembrolizumAb PD-1–targeted therapies have shown clinical activity in a wide variety of cancer types. However, not all patients experience clinical benefit from PD-1 therapy nor it is sensitive enough using ELISA for critical cancer sera antigen determination. We predict the UNIVmAb can be applied as a diagnostic tool for early and late cancers.
Acknowledgment
The authors acknowledge the Preethi Center of Oncology Logic and Clue diagnostic cent and K.R.Hospital for supplying normal and the cancer serum samples. We also thank the nurses at the center for drawing blood with the consent of the patients and the technicians who performed western blot and Elisa experiments. We appreciate Prof Tom Wight for consulting and going over the paper.
Competing Interests
The authors declare that they have no competing interests.
Authors Contribution
SDB made contributions to conception, design and interpretation. DM, VVI, NP performed the experiments, SAV, TW and SDB reviewed and commented on the manuscript. All the authors read and approved the manuscript.
Funding
Personal donation.
Research approval
The committee for the Preethi center of Oncology has approved the work. (MYS-00340-AA-NH).
References
- Manjunath D, Kumara SB, Venkata SA, et al. Validation and evaluation of a common biomarker in human cancers sera protein detected by a monoclonal antibody UNIVmAb. BMC Res. 2019;12:744.
- Baas P, Crino L, Eberhardt WE, et al. Nivolumab versus Docetaxel in advanced squamous-cell non-small-cell lung cancer. New Engl J Med. 2015;373:123-35.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in advanced renal-cell carcinoma. New Engl J Med. 2015;373: 1803-13.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in advanced nonsquamous non-small-cell lung cancer. New Engl J Med. 2015;373: 1627-39.
- Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. New Engl J Med. 2015;372:311-19.
- Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/ refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018;36:1428-39.
- Pagès F. Effector memory T cells, early metastasis, and survival in colorectal cancer. Engl J Med. 2005. 353; 2654-666.
- 8Angulo GD, Yuen C, Palla SL, et al. Absolute lymphocyte count is a novel prognostic indicator in ALL and AML. Cancer. 2008; 112:407-15.
- Simeone E. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer ImmunolImmunother. 2014;63:675-83.
- Wolpin BM, Richards DA, Cohn AL, et al: Performance of a blood-based test for the detection of multiple cancer types. Gastrointestinal Cancers Symposium. Abstract 283. 2020.
- Boregowda RK, Appaiah HN, Karunakumar M, et al. Development and validation of H11B2C2 monoclonal antibody-reactive hyaluronic acid-binding protein: overexpression of HABP during human tumor progression. Tumour Biol. 2013;34:597-608.
- Fekry B, Siddaiah M, Srinivas KP, et al. Purification of hyaluronan binding proteins from human normal and cancer serum Iran. J. Cancer Prev. 2010;3:79-86.
- Jung CS, Foerch C, Schanzer A, et al. Serum GFAP is a diagnostic marker for glioblastomamultiforme. Brain. 2007;130:3336-41.
- Koopmann J, Rosenzweig CN, Zhang Z, et al. Serum markers in patients with resectable pancreatic adenocarcinoma: macrophage inhibitory cytokine 1 versus CA19-9. Clin Cancer Res. 2006; 12:442-46.
- Duffy MJ. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol. 2010; 21:441-46.
- Fiedler GM. Resistance to Trastuzumab in Breast Cancer. Clin Can Res. 2009;15:3612-19.
- Chu D, Kohlmann W, Adler DG. Identification and screening of individuals at increased risk for pancreatic cancer with emphasis on known environmental and genetic factors and hereditary syndromes. JOP. 2010; 11:203-12.
- Toole B P. Hyaluronan and its binding proteins, the hyaladherins. Curr Opin Cell Biol, 2: 839-44.
- Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002; 277: 4589-89.
- Jackson DG. Immunological functions of hyaluronan and its receptors in the lymphatics. Immunol Rev;230:216-31 (2009).
- Kahmann JD, O’Brien R, Werner JM, et al. Localization and characterization of the hyaluronan-binding site on the Link module from human TSG-6. Structure. 2002; 8: 763-74.
- Liu N, Gao F, Han Z, et al. Hyaluronan synthase 3 overexpression pro¬motes the growth of TSU prostate cancer cells. Cancer Res. 2001; 61, 5207-5214.
- Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell DevBiol. 2007. 23:435-61.
- Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homo¬logue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. The J Cell Biol. 1999; 4:789-801.
- Banerjee SD, Toole BP. Monoclonal antibody to chick embryohyaluronan-binding protein: changes in the distribution of binding protein during early brain development. Dev Biol. 1991;146:186-197.
- Fekry B, Manjunath S, Kollapalli PS, et al. Purification of hyaluronan binding proteins from human normal and cancer serum. Iran J Can Prev. 2010;2:79-86.
- Huang L, Grammatikakis N, Yoneda M, et al. Molecular characterization of a novel intracellular hyaluronan-binding protein. J Biol Chem. 2000;275:29829-839.
- Banerjee SD, Thimmaiah KN, KarunaKumar M, et al.: Hyaluronic acid-binding protein, a common antigen over-expressed in all human tumor tissues: A possible marker of tumor progression. Am Assoc Cancer Res. 2000;295:1878.
- Kumar JK. Aronson AC, Pilko G, et al. Clinical evaluation of the TK 210 ELISA in sera from breast cancer patients demonstrates high sensitivity and specificity in all stages of the disease. Tum Biol. 2016;37:11937-11945.
- Ye GJ, Oshins RA, Rouhani FN, et al. Development, validation and use of ELISA for antibodies to human alpha-1 antitrypsin. J Immunol Meth. 2013;388:18-24.
- Maple L, Lathrop R, Bozich S, et al. Development and Validation of ELISA for Herceptin detection in human serum: J Immunol Meth 2004;295:169-82.
- Jaedicke KM, Taylor JJ, Preshaw PM. Validation and quality control of ELISAs for the use with human saliva samples: J Immunol Met. 2012;377:62-65.
- Selman ML, Brandt J, Palarasah Y, et al. An enzyme-linked immunosorbent assay (ELISA) for quantification of human collectin 11 (CL-11, CL-K1): J Immunol Met. 2012;375:182-88.
- Suwinski R, Giglok M, Galwas-Kliber K, et al. Blood serum proteins as biomarkers for prediction of survival, locoregional control and distant metastasis rate in radiotherapy and radio-chemotherapy for non-small cell lung cancer. BMC Canc. 2019;19:427-39.
- Blasco H, Lalmanach G, Godat E, et al. Evaluation of a peptide ELISA for the detection of rituximab in serum: J Immunol Met. 2007;325:127-39.
- Toole B P. Hyaluronan from extracellular glue to pericellular cue. Nat Rev Canc. 2004;4:528-39.
- Traeger L, Ellermann I, Weithoft H, et al. Serum Hepcidin and GDF-15 levels as prognostic markers ibUrothelial carcinoma of the upper urinary tract and renal cell carcinoma. BMC Canc. 2019;19:74-83.
- Baas P, Crino L, Eberhardt WE, et al. Nivolumab versus Docetaxel in advanced squamous-cell non-small-cell lung cancer. New Engl J Med. 2015;373(2):123-35.