Research Article - Biomedical Research (2017) Volume 28, Issue 3
Design, synthesis and calcium channel blocking activity of novel tetrahydropyrimidine derivatives for potential benefit in angina pectoris
Gang Liu*, Yan-yan Liu and Yong-cun HuDepartment of Cardiology, Anyang District Hospital, Anyang, Henan, China
Accepted date: July 27, 2016
Abstract
Aim: The present study deals with the discovery of some novel tetrahydropyrimidine derivatives for its possible effect on calcium channel blocking activity.
Methods: A novel series tetrahydropyrimidine derivatives were synthesized by simple and cost effective synthetic route. The structure of the synthesized derivatives was ascertained by the 1H-NMR, 12C-NMR, mass, FT-IR and elemental analyses.
Results: Results revealed that, designed compounds effectively antagonises the Ca2+ in A7r5 cell than SH-SY5Y cells suggesting its specific modulation of L-type calcium channel as A7r5 cells are highly expressed for L-type calcium channel. Moreover, the results has been further substantiated by molecular docking study, where, the most active analogue 6a has been docked with AID-β complex of L-type calcium channel and showed Ki of 23.73 μM. The estimated free energy of binding was found to be -6.31 kcal/mol.
Conclusion: In the present study, we have discovered a novel tetrahydropyrimidine derivative by simple and cost effective synthetic route which able to effectively antagonise the Ca2+ in SH-SY5Y and A7r5 cells in comparison to standard drug.
Keywords
Synthesis, Tetrahydropyrimidine derivatives, Docking, Calcium channel blocking, Angina pectoris.
Introduction
Being as a vital organ of a human body, the proper functioning of the heart is the major concern for the clinical fraternity due to changes in lifestyle which adversely affect it [1,2]. As a result, diseases affecting cardiovascular system are account for the leading cause of death and a major cause of disability worldwide [2,3]. Among several cardiovascular disorders, around 30% of patients are affected by angina pectoris [4]. It was considered as a condition resulting in chest pain owing to inadequate supply of blood to the heart [5]. Thus, the present clinical practice is depend upon reduction in pain, prevention of disease progression through risk reduction and antianginal therapies (beta-blockers, long-acting nitrates, calcium-channel blockers) [6,7]. In an excellent study, it has been found that antianginal agents provide symptomatic relief together with risk reduction. This has causes the high level of patients satisfaction and improved quality of life [8].
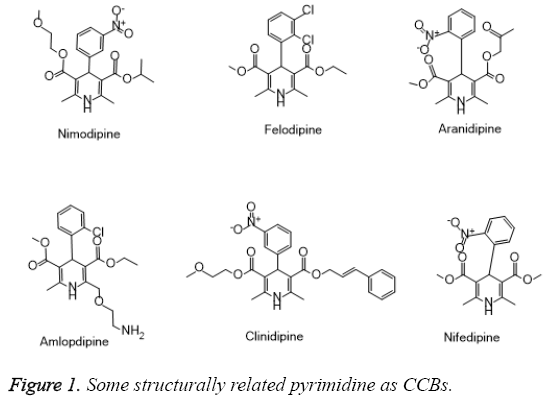
Concerning this, among the antianginal agents, Calcium Channel Blockers (CCBs) have attracted attention from large number of researchers towards the development of novel and effective agents [9]. The structurally related pyrimidines and 1,4-Dihydropyridine (1,4-DHP) belongs to a class of organic compounds which are known for its excellent antianginal activity (e.g., Nifedipine) [10,11]. Several studies have reported the discovery of advanced derivatives endowed with excellent bioactivity profile showed in Figure 1. Therefore, the present study deals with the discovery of some novel tetrahydropyrimidine derivatives for its possible effect on calcium channel blocking activity.
Materials and Methods
Chemistry
General procedure for synthesis of compounds 6-methyl-5- propionyl-4-(substituted)-3,4-dihydropyrimidin-2 (1H)-one (4): To a mixture of urea (1, 0.15 mol), substituted aldehydes (3, 0.1 mol) and ethylacetoacetate (2, 0.1 mol) in ethanol (75 ml), 4 drops of concentrated hydrochloric acid was added and heated for 1.5 h at 70˚C. The resulting reaction mixture was the poured into ice cold water (100 ml) with constant stirring and left overnight at room temperature. After that, it was filtered and residue was dried at room temperature. The final product (4) has been obtained by recrystallizing the crude product from ethanol [12].
General method for Synthesis of 5-(2-hydrazinylacetyl)-6- methyl-4-(substituted)-3,4-dihydropyrimidin-2 (1H)-one (5): To 3 mmol (0.14 ml) of boiling solution of hydrazine hydrate (99%), 1 mmol of above synthesized compound (4) in ethanol was added drop-wise with constant stirring and mixture was refluxed for 3.5 h. After the completion of reaction as ascertained by TLC, the reaction mixture was cooled to room temperature. and separated product was filtered. The filter cake containing crude product was washed with cold water and recrystallized from methanol to afford pure product 5.
General procedure for synthesis of title derivatives (6): To a stirred solution of compound 5 (2 g, 6.94 mmol) in acetonitrile (20 ml) was added (E)-(3- bromoprop-1-enyl)-benzene 6 (1.36 g, 6.9 mmol) and K2CO3 (1.92 g, 13.88 mmol) and a catalytic amount of tertiary butyl ammonium iodide (PTC) at room temperature. The resulting reaction mixture was refluxed for 10 h. After completion of the reaction as indicated by TLC, the solvent was removed in vacuo. The resulting residue was extracted twice with ethyl acetate (30 mL). The combined organic layers were washed with brine, dried over Na2SO4 and concentrated under reduced pressure to obtain the crude product, which was later purified with column chromatography.
N'-cinnamyl-4-(4-hydroxyphenyl)-6-methyl-2-oxo-1,2,3,4- tetrahydropyrimidine-5-carbohydrazide (6a): Yield: 75%; MP: 232-234˚C; MW: 378.42; Rf: 0.67; FT-IR (νmax; cm-1 KBr): 3325 (O-H str), 3187 (secondary N-H str), 3078 (Ar C-H str), 2986 (Ali C-H str), 2948 (C-H str of methyl), 1708 (C=O str), 1598 (NH-NH-CO str), 1612 (C=C), 781 cm-1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 8.07 (s, 1H, NH-CO), 7.40-7.24 (m, 5H, Ar-H), 7.06-6.63 (m, 4H, Ar-H), 6.48-6.37 (m, 2H, Alkene), 5.98 (s, 2H, NH X2), 5.38 (s, 1H, Ar-OH), 5.13 (s, 1H, Pyrimidine-H), 3.58 (s, 2H, CH2), 2.18 (s, 3H, CH3), 1.19 (s, 1H,NH); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.3, 156.7, 150.2, 146.2, 136.5, 135.8, 131.7, 128.7, 128.3, 127.8, 126.3, 124.4, 115.8, 108.7, 54.2, 53.6, 18.2; Mass: 379.47 (M+1); Elemental analysis for C21H22N4O3: Calculated: C-66.65; H-5.86; N-14.81; Found: C-66.58; H-5.93; N-14.73.
N'-cinnamyl-4-(3-hydroxyphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carbohydrazide (6b): Yield: 67%; MP: 241-243˚C; MW: 378.42; Rf: 0.62; FT-IR (νmax; cm-1 KBr): 3328 (O-H str), 3189 (secondary N-H str), 3072 (Ar C-H str), 2981 (Ali C-H str), 2943 (C-H str of methyl), 1704 (C=O str), 1596 (NH-NH-CO str), 1618 (C=C), 772 cm-1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 8.05 (s, 1H, NH-CO), 7.42-7.25 (m, 5H, Ar-H), 7.16-6.81 (m, 4H, Ar-H), 6.51-6.35 (m, 2H, Alkene), 5.97 (s, 2H, NH X2), 5.32 (s, 1H, Ar-OH), 5.09 (s, 1H, Pyrimidine-H), 3.63 (s, 2H, CH2), 2.21 (s, 3H, CH3), 1.23 (s, 1H, NH); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.3, 156.9, 150.3, 146.2, 144.8, 136.5, 131.6, 129.8, 128.7, 128.5, 127.9, 124.4, 119.7, 113.8, 112.7, 108.6, 54.5, 53.7, 18.1; Mass: 379.43 (M+1); Elemental analysis for C21H22N4O3: Calculated: C-66.65; H-5.86; N-14.81; Found: C-66.54; H-5.85; N-14.87.
N'-cinnamyl-4-(2-hydroxyphenyl)-6-methyl-2-oxo-1,2,3,4- tetrahydropyrimidine-5-carbohydrazide (6c): Yield: 71%; MP: 238-239˚C; MW: 378.42; Rf: 0.73; FT-IR (νmax; cm-1 KBr): 3335 (O-H str), 3183 (secondary N-H str), 3078 (Ar C-H str), 2985 (Ali C-H str), 2947 (C-H str of methyl), 1709 (C=O str), 1592 (NH-NH-CO str), 1613 (C=C), 762 cm-1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 8.09 (s, 1H, NH-CO), 7.43-7.24 (m, 5H, Ar-H), 7.09-6.83 (m, 4H, Ar-H), 6.56-6.28 (m, 2H, Alkene), 5.98 (s, 2H, NH X2), 5.37 (s, 1H, Ar-OH), 5.08 (s, 1H, Pyrimidine-H), 3.69 (s, 2H, CH2), 2.26 (s, 3H, CH3), 1.22 (s, 1H, NH); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.3, 154.2, 150.4, 146.4, 136.5, 131.6, 128.7, 128.5, 128.3, 128.1, 127.8, 124.4, 122.9, 121.2, 115.6, 108.7, 53.7, 47.9, 18.4; Mass: 379.38 (M+1); Elemental analysis for C21H22N4O3: Calculated: C-6.65; H-5.86; N-14.81; Found: C-66.59; H-5.89; N-14.75.
N'-cinnamyl-4-(4-fluorophenyl)-6-methyl-2-oxo-1,2,3,4- tetrahydropyrimidine-5-carbohydrazide (6d): Yield: 76%; MP: 248-249˚C; MW: 380.42; Rf: 0.71; FT-IR (νmax; cm-1 KBr): 3189 (secondary N-H str), 3074 (Ar C-H str), 2987 (Ali C-H str), 2949 (C-H str of methyl), 1712 (C=O str), 1597 (NHNH- CO str), 1614 (C=C), 1158 (C-F str), 761 cm-1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 8.05 (s, 1H, NH-CO), 7.41-7.26 (m, 5H, Ar-H), 7.21-7.09 (m, 4H, Ar-H), 6.58-6.25 (m, 2H, Alkene), 5.96 (s, 2H, NH X2), 5.09 (s, 1H, Pyrimidine-H), 3.72 (s, 2H, CH2), 2.24 (s, 3H, CH3), 1.24 (s, 1H, NH); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.3, 160.8, 150.3, 146.2, 138.9, 136.5, 131.6, 128.7, 128.5, 127.8, 124.4, 115.5, 108.7, 54.2, 53.7, 18.2; Mass : 381.47 (M+1); Elemental analysis for C21H21FN4O2: Calculated: C-66.30; H-5.56; N-14.73; Found: C-66.35; H-5.52; N-14.68.
N'-cinnamyl-4-(3-fluorophenyl)-6-methyl-2-oxo-1,2,3,4- tetrahydropyrimidine-5-carbohydrazide (6e): Yield: 68%; MP: 258-257˚C; MW: 380.42; Rf: 0.63; FT-IR (νmax; cm-1 KBr): 3193 (secondary N-H str), 3079 (Ar C-H str), 2984 (Ali C-H str), 2952 (C-H str of methyl), 1714 (C=O str), 1595 (NHNH- CO str), 1612 (C=C), 1163 (C-F str), 765 cm-1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 8.07 (s, 1H, NH-CO), 7.43-7.24 (m, 5H, Ar-H), 7.31-6.78 (m, 4H, Ar-H), 6.56-6.21 (m, 2H, Alkene), 5.98 (s, 2H, NH X2), 5.11 (s, 1H, Pyrimidine- H), 3.75 (s, 2H, CH2), 2.23 (s, 3H, CH3), 1.28 (s, 1H,NH); 13CNMR (100 MHz, CDCl3) δ, ppm: 165.3, 162.8, 150.3, 146.2, 144.8, 136.5, 131.4, 130.2, 128.6, 128.5, 127.9, 124.2, 122.3, 113.8, 113.5, 108.6, 54.2, 53.6, 18.2; Mass : 381.38 (M+1); Elemental analysis for C21H21FN4O2: Calculated: C-66.30; H-5.56; N-14.73; Found: C-66.34; H-5.59; N-14.76.
N'-cinnamyl-4-(2-fluorophenyl)-6-methyl-2-oxo-1,2,3,4- tetrahydropyrimidine-5-carbohydrazide (6f): Yield: 71%; MP: 254-255˚C; MW: 380.42; Rf: 0.69; FT-IR (νmax; cm-1 KBr): 3196 (secondary N-H str), 3082 (Ar C-H str), 2987 (Ali C-H str), 2959 (C-H str of methyl), 1712 (C=O str), 1598 (NHNH- CO str), 1618 (C=C), 1169 (C-F str), 761 cm-1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 8.09 (s, 1H, NH-CO), 7.44-7.23 (m, 5H, Ar-H), 7.56-7.10 (m, 4H, Ar-H), 6.58-6.18 (m, 2H, Alkene), 5.97 (s, 2H, NH X2), 5.09 (s, 1H, Pyrimidine-H), 3.73 (s, 2H, CH2), 2.25 (s, 3H, CH3), 1.24 (s, 1H,NH); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.2, 159.5, 150.3, 146.2, 136.5, 131.6, 129.4, 128.7, 128.5, 128.3, 127.8, 124.3, 124.2, 115.2, 108.7, 53.7, 47.4, 18.2; Mass : 381.43 (M +1); Elemental analysis for C21H21FN4O2: Calculated: C-66.30; H-5.56; N-14.73; Found: C-66.27; H-5.58; N-14.79.
4-(4-chlorophenyl)-N'-cinnamyl-6-methyl-2-oxo-1,2,3,4- tetrahydropyrimidine-5-carbohydrazide (6g): Yield: 67%; MP: 242-243˚C; MW: 396.87; Rf: 0.64; FT-IR (νmax; cm-1 KBr): 3194 (secondary N-H str), 3086 (Ar C-H str), 2982 (Ali C-H str), 2964 (C-H str of methyl), 1718 (C=O str), 1596 (NHNH- CO str), 1613 (C=C), 821 (C-Cl str), 765 cm-1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 8.06 (s, 1H, NH-CO), 7.42-7.25 (m, 5H, Ar-H), 7.38-7.34 (m, 4H, Ar-H), 6.57-6.17 (m, 2H, Alkene), 5.98 (s, 2H, NH X2), 5.11 (s, 1H, Pyrimidine- H), 3.75 (s, 2H, CH2), 2.23 (s, 3H, CH3), 1.28 (s, 1H,NH); 13CNMR (100 MHz, CDCl3) δ, ppm: 165.3, 150.4, 146.2, 141.5, 136.5, 132.4, 131.6, 128.8, 128.3, 127.9, 126.2, 124.4, 108.7, 54.2, 53.6, 18.2; Mass: 397.92 (M+1); Elemental analysis for C21H21ClN4O2: Calculated: C-63.55; H-5.33; N-14.12; Found: C-63.50; H-5.37; N-14.15.
4-(3-chlorophenyl)-N'-cinnamyl-6-methyl-2-oxo-1,2,3,4- tetrahydropyrimidine-5-carbohydrazide (6h): Yield: 58%; MP: 264-265˚C; MW: 396.87; Rf: 0.67; FT-IR (νmax; cm-1 KBr): 3196 (secondary N-H str), 3087 (Ar C-H str), 2981 (Ali C-H str), 2967 (C-H str of methyl), 1712 (C=O str), 1594 (NHNH- CO str), 1612 (C=C), 825 (C-Cl str), 762 cm-1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 8.08 (s, 1H, NH-CO), 7.44-7.23 (m, 5H, Ar-H), 7.42-7.11 (m, 4H, Ar-H), 6.56-6.21 (m, 2H, Alkene), 5.98 (s, 2H, NH X2), 5.09 (s, 1H, Pyrimidine-H), 3.78 (s, 2H, CH2), 2.25 (s, 3H, CH3), 1.26 (s, 1H, NH); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.3, 150.2, 146.2, 144.7, 136.4, 134.2, 131.6, 129.8, 128.4, 128.3, 127.9, 126.8, 126.7, 125.2, 124.4, 108.7, 53.6, 53.5, 18.1; Mass: 397.89 (M+1); Elemental analysis for C21H21ClN4O2: Calculated: C-63.55; H-5.33; N-14.12; Found: C-63.59; H-5.32; N- 14.17.
4-(2-chlorophenyl)-N'-cinnamyl-6-methyl-2-oxo-1,2,3,4- tetrahydropyrimidine-5-carbohydrazide (6i): Yield: 75%; MP: 267-268˚C; MW: 396.87; Rf: 0.74; FT-IR (νmax; cm-1 KBr): 3192 (secondary N-H str), 3085 (Ar C-H str), 2987 (Ali C-H str), 2968 (C-H str of methyl), 1714 (C=O str), 1595 (NHNH- CO str), 1618 (C=C), 821 (C-Cl str), 769 cm ; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 8.09 (s, 1H, NH-CO), 7.65-7.17 (m, 4H, Ar-H), 7.41-7.26 (m, 5H, Ar-H), 6.57-6.23 (m, 2H, Alkene), 5.97 (s, 2H, NH X2), 5.12 (s, 1H, Pyrimidine-H), 3.74 (s, 2H, CH2), 2.27 (s, 3H, CH3), 1.28 (s, 1H, NH); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.3, 150.2, 146.2, 142.9, 136.5, 132.3, 131.5, 128.7, 128.4, 128.3, 128.1, 127.9, 126.7, 124.4, 108.7, 53.6, 49.1, 18.1; Mass: 397.85 (M +1); Elemental analysis for C21H21ClN4O2: Calculated: C-63.55; H-5.33; N-14.12; Found: C-63.51; H-5.29; N-14.15.
N'-cinnamyl-6-methyl-4-(4-nitrophenyl)-2-oxo-1,2,3,4- tetrahydropyrimidine-5-carbohydrazide (6j): Yield: 82%; MP: 286-287˚C; MW: 407.16; Rf: 0.78; FT-IR (νmax; cm-1 KBr): 3196 (secondary N-H str), 3086 (Ar C-H str), 2983 (Ali C-H str), 2969 (C-H str of methyl), 1718 (C=O str), 1597 (NHNH- CO str), 1528 (NO2 str), 1619 (C=C), 761 cm-1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 8.15-7.51 (m, 4H, Ar-H), 8.07 (s, 1H, NH-CO), 7.43-7.25 (m, 5H, Ar-H), 6.54-6.26 (m, 2H, Alkene), 5.98 (s, 2H, NH X2), 5.09 (s, 1H, Pyrimidine-H), 3.76 (s, 2H, CH2), 2.26 (s, 3H, CH3), 1.24 (s, 1H, NH); 13CNMR (100 MHz, CDCl3) δ, ppm: 165.3, 150.2, 149.5, 146.2, 145.9, 136.5, 131.6, 128.7, 128.5, 128.3, 127.9, 125.2, 124.4, 108.7, 54.1, 53.5, 18.1; Mass: 408.21 (M+1); Elemental analysis for C21H21N5O4: Calculated: C-61.91; H-5.20; N-17.19; Found: C-61.95; H-5.26; N-14.16.
N'-cinnamyl-6-methyl-4-(3-nitrophenyl)-2-oxo-1,2,3,4- tetrahydropyrimidine-5-carbohydrazide (6k): Yield: 72%; MP: 292-294˚C; MW: 407.16; Rf: 0.71; FT-IR (νmax; cm-1 KBr): 3192 (secondary N-H str), 3087 (Ar C-H str), 2989 (Ali C-H str), 2962 (C-H str of methyl), 1711 (C=O str), 1597 (NHNH- CO str), 1523 (NO2 str), 1617 (C=C), 765 cm-1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 8.13-7.59 (m, 4H, Ar-H), 8.09 (s, 1H, NH-CO), 7.42-7.26 (m, 5H, Ar-H), 6.57-6.28 (m, 2H, Alkene), 5.97 (s, 2H, NH X2), 5.12 (s, 1H, Pyrimidine-H), 3.78 (s, 2H, CH2), 2.24 (s, 3H, CH3), 1.27 (s, 1H, NH); 13CNMR (100 MHz, CDCl3) δ, ppm: 165.2, 150.3, 147.8, 146.2, 144.3, 136.5, 133.1, 131.6, 129.5, 128.7, 128.4, 127.9, 124.4, 121.9, 120.7, 108.6, 53.6, 53.1, 18.2; Mass: 408.19 (M+1); Elemental analysis for C21H21N5O4: Calculated: C-61.91; H-5.20; N-17.19; Found: C-61.93; H-5.16; N-14.23.
N'-cinnamyl-6-methyl-4-(2-nitrophenyl)-2-oxo-1,2,3,4- tetrahydropyrimidine-5-carbohydrazide (6l): Yield: 64%; MP: 279-281˚C; MW: 407.16; Rf: 0.75; FT-IR (νmax; cm-1 KBr): 3194 (secondary N-H str), 3089 (Ar C-H str), 2981 (Ali C-H str), 2967 (C-H str of methyl), 1718 (C=O str), 1595 (NHNH- CO str), 1521 (NO2 str), 1619 (C=C), 767 cm-1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 8.07 (s, 1H, NH-CO), 7.95-7.49 (m, 4H, Ar-H), 7.45-7.28 (m, 5H, Ar-H), 6.56-6.25 (m, 2H, Alkene), 5.98 (s, 2H, NH X2), 5.09 (s, 1H, Pyrimidine-H), 3.75 (s, 2H, CH2), 2.21 (s, 3H, CH3), 1.28 (s, 1H, NH); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.3, 150.2, 147.3, 146.1, 140.5, 136.4, 131.5, 130.8, 128.7, 128.5, 127.9, 127.7, 127.4, 124.8, 124.2, 108.7, 53.6, 49.5, 18.2; Mass: 408.17 (M+1); Elemental analysis for C21H21N5O4: Calculated: C-61.91; H-5.20; N-17.19; Found: C-61.87; H-5.23; N-14.18.
N'-cinnamyl-6-methyl-2-oxo-4-phenyl-1,2,3,4- tetrahydropyrimidine-5-carbohydrazide (6m): Yield: 77%; MP: 218-219˚C; MW: 362.42; Rf: 0.62; FT-IR (νmax; cm-1 KBr): 3191 (secondary N-H str), 3094 (Ar C-H str), 2985 (Ali C-H str), 2962 (C-H str of methyl), 1714 (C=O str), 1597 (NHNH- CO str), 1621 (C=C), 761 cm-1; 1H-NMR (400 MHz, DMSO, TMS) δ ppm: 8.09 (s, 1H, NH-CO), 7.41-7.24 (m, 5H, Ar-H), 7.33-7.21 (m, 4H, Ar-H), 6.54-6.21 (m, 2H, Alkene), 5.97 (s, 2H, NH X2), 5.11 (s, 1H, Pyrimidine-H), 3.78 (s, 2H, CH2), 2.24 (s, 3H, CH3), 1.26 (s, 1H, NH); 13C-NMR (100 MHz, CDCl3) δ, ppm: 165.2, 150.3, 146.1, 143.4, 136.5, 131.6, 128.7, 128.2, 127.9, 126.8, 126.4, 124.3, 108.7, 54.2, 53.5, 18.1; Mass: 363.45 (M+1); Elemental analysis for C21H22N4O2: Calculated: C-69.59; H-6.12; N-15.46; Found: C-69.63; H-6.09; N-15.46.
Pharmacology
Effect on Intracellular Ca2+
The Fluo-4 NW Calcium Assay Kit was obtained from Invitrogen (Sweden), whereas, the other kits were procured from Sigma-Aldrich (St Louis, MO, USA). The A7r5 (rat aorta smooth muscle cells) were obtained from the ATTC (USA) and SH-SY5Y (human neuroblastoma cell line) from the European Collection of Animal Cell Cultures (ECACC, UK). The cells were allowed to be grown at 37˚C in 5% CO2/95% air in DMEM, containing 2 mM of glutamine and supplemented with 10% FBS. The cells were passaged once a week using 0.25% trypsin, 0.53 mM EDTA solution until its confluence reached to desired level. The SH-SY5Y cells were taken into 96-well plates at a density of 30,000 cells per well and incubated for 24 h. whereas, the A7r5 cells were plated into 96-well plates at a density of 10,000 cells per well and incubated for 72 h. Thus, the changes in intracellular Ca2+ concentration were studied using the Fluo-4 NW Calcium Assay Kit. For this, the SHSY5Y and A7r5 cells were loaded with Fura-4NW for 45 min, then, the cells were pre-incubated in the dark for 15 min with the compounds to be tested (in DMSO) at concentrations from 0.8 to 100 mM. To induce the intracellular level of Ca2+, carbachol (100 nM) was in SH-SY5Y, whereas A7r5 cells were treated with 1.5 mM CaCl2 and KCl (50 mM) for 5 min. The intracellular Ca2+ concentration was measured using the fluorescence spectrophotometer with an excitation at 494 nm and an emission at 516 nm using amlodipine and nimodipine as standard. The IC50 values as mean ± SD were calculated using Graph Pad Prism 3.0 software.
Docking Study
Docking calculations were carried out using Docking Server [13]. Gasteiger partial charges were added to the ligand atoms. Non-polar hydrogen atoms were merged, and rotatable bonds were defined. Docking calculations were carried out on voltage-dependent calcium channel beta subunit functional core in complex with alpha1 interaction domain protein model. Essential hydrogen atoms, Kollman united atom type charges [14], and solvation parameters were added with the aid of Auto Dock tools [15]. Affinity (grid) maps of 60 × 60 × 60 Å grid points and 0.375 Å spacing were generated using the Auto grid program [15]. Auto Dock parameter set- and distancedependent dielectric functions were used in the calculation of the van der Waals and the electrostatic terms, respectively. Docking simulations were performed using the Lamarckian Genetic Algorithm (LGA) and the Solis and Wets local search method [16]. Initial position, orientation, and torsions of the ligand molecules were set randomly. All rotatable torsions were released during docking. Each docking experiment was derived from 10 different runs that were set to terminate after a maximum of 250000 energy evaluations. The population size was set to 150. During the search, a translational step of 0.2 Å, and quaternion and torsion steps of 5 were applied.
Results and Discussion
Chemistry
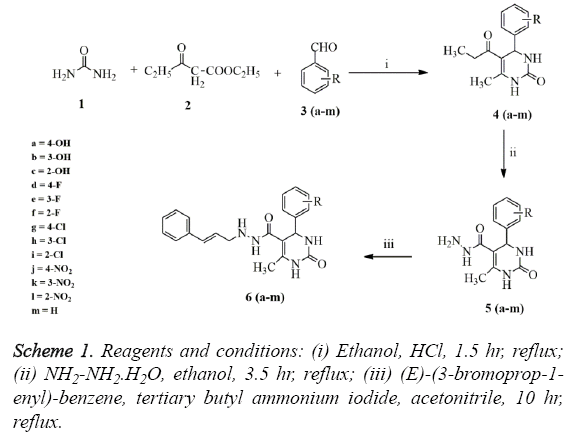
The target compound was achieved via simple cost effective synthetic route as shown in scheme 1. The reaction has been started with synthesis of DHP nucleus via classical Biginelli one-pot condensation reaction, where the reaction has been initiated using urea (1) ethyl acetoacetate (2) and substituted aldehyde derivatives (3) to afford substituted tetra hydro pyrimidines under reflux (4). The later compound was again allowed to react with hydrazine to furnish compound 5. The last step corresponds to the reaction between (E)-(3- bromoprop-1-enyl)-benzene and compound 5 in the presence of catalytic amount of tertiary butyl ammonium iodide (PTC) to afford final target derivatives 6 (a-m). The entire synthesized compounds were obtained in excellent yield ranging from 62-92%.
The structures of the synthesized final derivatives were ascertained by the 1H-NMR, 12C-NMR, mass, FT-IR and elemental analysis. FT-IR spectra of all derivatives 6 (a-m) were characterized by the appearance of a strong bands at 3183-3396 cm-1 attributed to secondary N-H group. Many strong absorption bands appear at 3072-3087 cm-1 which confirms the existence of C-H group of aromatic ring. The band corresponds to C=O pyrimidine ring appears at 1704-1718 cm-1. Furthermore, another band at 3325 cm-1 attributed to the stretching vibrations of the aromatic OH group. The NO2 group of aromatic ring appears at 1528 cm-1. Furthermore, another band at 1163 cm-1 attributed to the stretching vibrations of the aromatic F group. The 1H NMR spectrums of final derivatives 6 (a-m) reveal a multiplet corresponding to phenyl ring at 7.43-7.24 ppm. The pyrimidine NH proton appears at 8.07-8.09 ppm. The resonance due to pyrimidine substituted methyl proton found in the range of 2.21-2.28 ppm. Finally, all the structure of synthesized derivatives 6 (a-m) was confirmed by mass spectra and elemental analysis.
Moreover, various spectroscopic analyses were also utilized for the confirmation of structures of novel derivatives.
Pharmacological activity
Effect of calcium channel blocking activity in SHSY5Y and A7r5 cell lines: The interference of L- and N-type calcium channel was worked out as mechanism of action of classical antianginal drugs belonging to the chemical class of structurally related pyridines. Thus, it is imperative to determine the effect of above synthesized compounds on both L-type and N-type calcium channel. Consequently, the present study utilizes the neuroblastoma SH-SY5Y cells (expressing Land N-type Ca2+ channels) and the rat aorta smooth muscle A7r5 cells, (containing only L-type Ca2+ channels) to determine the effect of target compounds on the intracellular level of Ca2+. The results had been present in Table 1 along with amlodipine and nimodipine as the positive controls.
| Compound | Ca2+ Antagonism, IC50 (in µM)* | |
|---|---|---|
| In SH-SY5Y cells | In A7r5 cells | |
| 6a | 15 ± 3 | 0.067 ±0.0045 |
| 6b | 21 ± 7 | 1.56 ± 0.0023 |
| 6c | 45 ± 5 | 6.45 ± 0.031 |
| 6d | 80 ± 7 | >100 |
| 6e | 85 ± 2 | >100 |
| 6f | >100 | >100 |
| 6g | 56 ± 4 | 8.5 ± 0.065 |
| 6h | 67 ± 1 | 14.3 ± 0.015 |
| 6i | 81 ± 3 | 19.4 ± 0.033 |
| 6j | NA | NA |
| 6k | NA | NA |
| 6l | NA | NA |
| 6m | NA | NA |
| Amlodipine | 13 ± 3 | 0.242 ± 0.032 |
| Nimodipine | 61 ± 5 | 0.013 ± 0.0026 |
Table 1: Effect of compound 6 (a-m) on the in SH-SY5Y and A7r5 cells.
The results of the investigation revealed that, entire set of derivatives exhibit considerable inhibition of Ca2+ in both the cell lines except 6j, 6k, 6l and 6m. The compound 6a, 6b and 6c having varying substitution of hydroxyl group showed excellent inhibition of Ca+2 concentration in SH-SY5Y cells. It has been found that, compound 6a having p-substitution found more active then m- and o-substituted derivative, compounds 6b and 6c, respectively. Moreover, the activity was greatly diminished upon the introduction of halogen electron withdrawing group, 6d-I which showed weak calcium antagonistic activity. Among the halogen derivative, chloro containing (6g, 6h and 6i) compounds exhibit high potency over fluoro-substituted derivative (6d, 6e and 6f). It was surprising to note that, NO2 containing compounds (6j, 6k and 6l) exhibit no activity at the highest tested concentration in both the cells. Moreover, non-substituted compound (6m) also showed no activity. In respect of effect on A7r5 cells, the same pattern of calcium antagonistic activity was observed revealing compound 6a, 6b and 6c as most effective analogues than other tested derivatives. The mild to moderate activity was revealed by compound 6g, 6h and 6i in A7r5 cells. It was surprising to note that, the antagonistic properties of most active compounds were found similar to that of Amlodipine and Nimodipine as standard.
Result of the comparative inhibitory profile as shown in Table 1 confirmed that, compounds predominantly antagonises the effect Ca2+ ions in A7r5 cells in comparison to SH-SY5Y cells. This suggests that, these compounds effectively modulate the L-type calcium channel which is found highly expressed in A7r5 cells. The structure-activity relationship study suggests that, the activity of compounds has been greatly reduced by the presence of electron withdrawing group. Whereas, the activity has been significantly improved by the presence of hydroxyl group. The positions of substituted group have found to be considerable influence on the bioactivity of the compounds. The compounds containing para-substituted derivative was found more active than, meta- and ortho-substituted derivatives.
Docking study with L-type Ca2+ channel
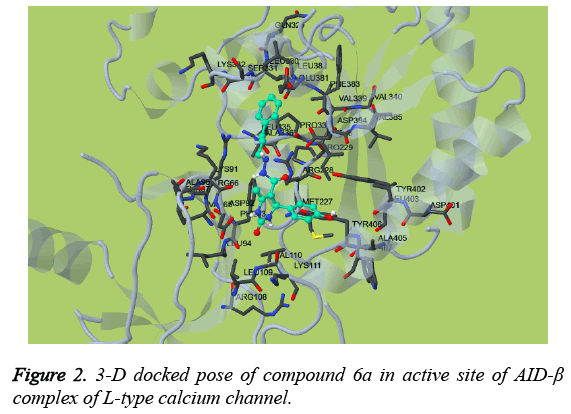
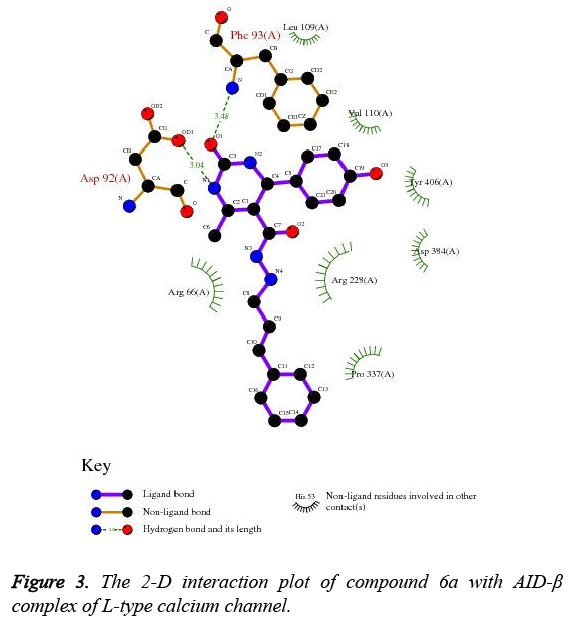
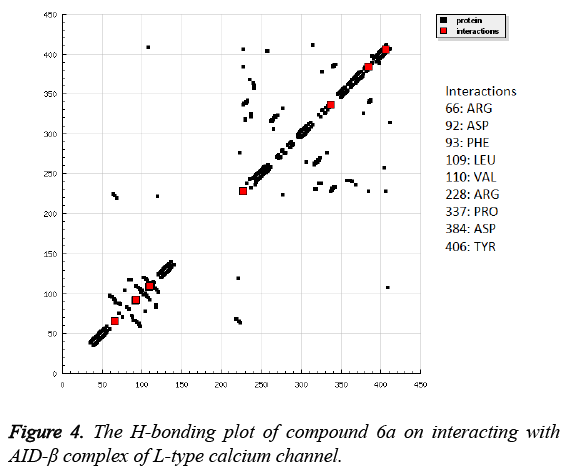
Impressed by the exceptional Ca2+ antagonistic activity of designed target molecules, it is imperative to understand the molecular mechanism behind this. From the above study, it has been suggested that, designed target molecules effective antagonises the Ca2+ in the A7r5 cells which is mediated by inhibition of L-type calcium channel. Therefore, the molecular docking study was undertaken to elucidate the key structural contact between the AID-β complexes of L-type calcium channel obtained from PDB with ID: 1T3L. The docking study revealed that, compound 6a efficiently docked in the active site of AID-β complex of L-type calcium channel as shown in Figure 2. It has been found that, compound 6a engage neighbouring residues of AID-β complex of L-type calcium channel by forming H-bonds with Asp92 and Phe93. The compound 6a was also revealed to create pi-pi interaction with Tyr406. The other non-bonded interaction with Phe93 via creating cation-pi interaction. The detailed 2-D ligand-protein interaction has been shown in Figure 3 along with HB Plot in Figure 4. The interaction of compound 6a was found in accordance with the scoring parameters as presented in Table 2, which confirmed that it can be able to proficiently able to engage in neighbouring amino acids residues present in the active site. The compound 6a showed estimated inhibition constant of (Ki) of 23.73 μM along with -6.31 kcal/mol suggesting its excellent affinity for the AID-β complex of Ltype calcium channel.
| S No. | Estimated free energy of binding | Estimated inhibition constant (Ki) |
vdW+H-bond+desolv energy | Electrostatic energy | Total intermolecular energy |
|---|---|---|---|---|---|
| -6.31 kcal/mol | 23.73 µM | -8.52 kcal/mol | -0.06 kcal/mol | -8.58 kcal/mol |
Table 2: Scoring profile of compound 6a in of AID-β complex of L-type calcium channel.
Conclusion
In the present study, we have discovered novel derivatives of tetrahydropyrimidine by simple and cost effective synthetic route which able to effectively antagonise the Ca2+ in SHSY5Y and A7r5 cells in comparison to standard drug. Results revealed that, designed compounds effectively antagonises the Ca2+ in A7r5 cell than SH-SY5Y cells suggesting effective and specific modulation of L-type calcium channel as A7r5 cells are highly expressed for L-type calcium channel. Moreover, the results has been further substantiated by molecular docking study, where most active analogue 6a has been docked with AID-β complex of L-type calcium channel. The docked pose of compound 6a showed, it was efficiently docked with AID-β complex of L-type calcium channel with Ki of 23.73 μM and estimated free energy of binding of -6.31 kcal/mol. It also showed the formation of various H-bond and non-covalent interaction like pi-pi and pi-cation with AID-β complex of Ltype calcium channel.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Adib F, Mao H, Kabelac Z, Katabi D, Miller RC. Smart homes that monitor breathing and heart rate. Annu ACM Conf Hum Factors Comput Syst-CHI 2015; 15: 837-846.
- Diener E, Oishi S, Lucas RE. Personality, culture, and subjective well-being- emotional and cognitive evaluations of life. Annu Rev Psychol 2003; 54: 403-425.
- World Health Organization. Cardiovascular diseases. Fact sheet 2012; 2012: 4-7.
- Gowda RM, Khan IA, Punukollu G, Vasavada BC, Nair CK. Treatment of refractory angina pectoris. Int J Cardiol 2005; 101: 1-7.
- Birmingham CL, Stigant C, Goldner EM. Chest pain in anorexia nervosa. Int J Eat Disord 1999; 25: 219-222.
- Cohn PF. Concomitant use of nitrates, calcium channel blockers, and beta blockers for optimal antianginal therapy. Clin Cardiol 1994; 17: 415-421.
- Heidenreich PA, McDonald KM, Hastie T, Fadel B, Hagan V, Lee BK, Hlatky MA. An evaluation of beta-blockers, calcium antagonists, nitrates, and alternative therapies for stable angina. Datab Abstr Rev Eff 2014; 1-5.
- Spertus JA, Dewhurst T, Dougherty CM, Nichol P. Testing the effectiveness of converting patients to long-acting antianginal medications-The Quality of Life in Angina Research Trial (QUART). Am Heart J 2001; 141: 550-558.
- Godfraind T. Calcium channel blockers in cardiovascular pharmacotherapy.J. Cardiovasc Pharmacol Ther 2014; 19: 501-515.
- Grabner M, Wang Z, Hering S, Striessnig J, Glossmann H. Transfer of 1,4-dihydropyridine sensitivity from L-type to class A (BI) calcium channels. Neuron. 1996; 16: 207-218.
- Triggle DJ. 1,4-Dihydropyridines as calcium channel ligands and privileged structures. Cell Mol Neurobiol 2003; 23: 293-303.
- Narkhede HI, Nevagi RJ, Kumbhare M, Kaur P. Synthesis and in-vitro screening of novel dihydropyrimidine derivatives as potential calcium channel blockers. Der Pharm Chemica 2014; 6:221-227
- Bikadi Z, Hazai E. Application of the PM6 semi-empirical method to modeling proteins enhances docking accuracy of AutoDock J Cheminf 2009; 1: 15.
- Halgren TA. Merck molecular force field, Basis, form, scope, parametrization, and performance of MMFF94. J Comput Chem 1998; 17: 490-519.
- Morris GM, Goodsell DS. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 1998; 19: 1639-1662.
- Solis FJ, Wets RJB. Minimization by Random Search Techniques. Math Oper Res 1981; 6: 19-30.