Review Article - Biomedical Research (2023) Volume 34, Issue 3
Dengue as an emerging infection and co-infections: An update.
Shridhar Pattar, Santhosh SC, Prasan Kumar Panda*, Prabhat Rijal
Department of Internal Medicine (Infectious Disease Division), All India Institute of Medical Sciences, Rishikesh, India
- Corresponding Author:
- Prasan Kumar Panda
Department of Internal Medicine (Infectious Disease Division)
All India Institute of Medical Sciences
Rishikesh India
Accepted date: June 14, 2023
Abstract
Dengue is a viral infection, transmitted by the Aedes mosquito and affects millions of people worldwide each year. With no specific treatment or vaccine available, prevention and control of the disease rely heavily on public health measures such as vector control and early diagnosis. In recent years, the incidence of dengue has been on the rise, with increasing numbers of severe cases and fatalities reported. This has prompted extensive research efforts to better understand the virus and develop effective strategies for prevention and treatment. In this review article, we provide an update on the current state of knowledge on dengue and their co-infections, including the epidemiology, pathogenesis, clinical manifestations, diagnosis, treatment, and prevention strategies. We also highlight the latest research findings and ongoing efforts to develop new interventions for this significant global health challenge
Keywords
Epidemiology, Pathogenesis, Clinical manifestations, Dengue.
Introduction
The global burden of dengue is estimated to be 1.04 million cases as in 2017 and the incidence rate is 1371 per 1 lakh population [1]. Day by day, its emergence is increasing with globalization. The overall seroprevalence of dengue infection in India is 48.7%. It differs significantly between various regions of the country: The highest seroprevalence is in Southern areas (76.9%), followed by Western (62.3%), and Northern (60.3%) regions. The seroprevalence is higher in urban (70.9%) than in rural areas (42.3%) [2]. In the state of Uttarakhand India, there has been quite a significant fluctuation in the number of dengue cases in the recent few years. The state reported 10,622 cases in the year 2019 which decreased to only 76 in 2020.
The capillary leak phenomenon is the key for clinical presentation and for management by fluid therapy. Nonavailability of effective vaccines, precise therapeutic agents, and efficient vector-control methods makes it challenging to control these days. This review focusses on recent updates on epidemiology, pathophysiology, clinical presentation, diagnostic modalities, co-infections, treatment, and preventive strategies.
Literature Review
Epidemiology
Dengue is a vector-borne viral disease caused by the flavivirus Dengue Virus (DENV) which is a positivestranded enveloped RNA virus. There are four distinct antigenic serotypes of the same, DENV-1 to DENV-4. A fifth serotype of the virus has also been reported [3]. All the four serotypes of DENV are prevalent in India. Of the four serotypes, DENV1 and DENV2 are prevalent in the northern region and DENV1, 2, and 3 are prevalent in the southern region of India. Multi-serotypic infection is commoner in all parts of India rather than monotypic infection [4].
Host factors: There are no specific host-related factors that predispose to the infection. However, chronic illnesses like asthma, sickle cell anemia, and diabetes mellitus predispose the patient to develop severe forms of the disease once infected. The virus can evade innate immune pathways to cause severe disease [5]. Although primary infection leads to immune response activation, the severity of infection is enhanced by heterotypic infection by different serotypes by the phenomenon of antibody-dependent enhancement [6].
Environmental factors: Environmental factors play a crucial role in any vector-borne disease. In the context of dengue infection, rainfall, temperature, and humidity have been associated with a higher incidence of infection [7,8]. The most common breeding sites for Aedes mosquitoes are artificial water containers like a bucket, tanks, bottles, etc.
Vectors and transmission: Dengue is transmitted by female mosquitoes mainly-Aedes aegypti and Aedes albopictus. These species share several characteristics making them successful invaders [9] (Table 1). A. aegypti usually breed in and around human households and lay eggs in both artificial containers as well as natural water reservoirs.
| Aedes aegypti | Aedes albopictus |
|---|---|
| It is the principal vector [10]. | It is also a competent vector [10]. |
| They frequently feed multiple hosts and are a discordant species-that takes multiple feeds in one gonotrophic cycle [11]. | It is also an aggressive feeder but feeds less frequently and is a concordant species-completes gonotrophic cycle in a single blood meal [11]. |
| Widespread distribution around the world, with predominance in tropical and subtropical countries [12]. | Distributed predominantly along the temperate climate [12]. |
| More efficient in transmitting DENV. | Less efficient. |
| Predominantly inhabits urban area [13]. | Predominantly inhabits rural area [13]. |
| Endophilic and endophagic [11]. | Endophilic but exophagic; an opportunistic feeder [11]. |
Table 1: Epidemiological differentiations of dengue-causing mosquitoes.
Patterns of transmission: The extrinsic incubation period for dengue infection is 7-14 days at 25-30°C [14]. Two types of transmission cycles have been noted, human transmission cycle in urban settings and sylvatic transmission cycles in forested areas. Transovarial transmission is essential for the continuation of both the cycles during the inter-epidemic period. Non-vector transmission routes in the form of transfusion of blood products, bone marrow transplant, needle stick injury, mucocutaneous route, and perinatal transmission have also been described in literature. Enhanced globalization has contributed to rapid expansion of DENV [15]. Dengue infection follows two patterns-epidemic dengue and hyperendemic dengue. The difference being single strain of DENV responsible in the former pattern while circulation of various serotypes in a community in the later one.
Pathophysiology
Pathogenesis of dengue infection and severe dengue is not yet fully established. Dengue virus targets macrophages, dendritic cells, mast cells, monocytes, hepatocytes and endothelial cells which eventually leads to vascular leak phenomenon, characteristic of dengue infection [16].
DENV entry into cells is facilitated by an unknown cell-surface receptor and can be affected by various glycoproteins. The E protein of the virus plays a significant role in receptor binding and membrane fusion, which allows the viral nucleocapsid to enter the cytoplasm and release the genome. The input positive-strand viral RNA is then translated into a single polyprotein and cleaved into individual structural and Non Stuctural (NS) proteins. The viral replication complex accumulates within vesicle packets or smooth membrane structures in the cytosol, and NS3, along with its cofactor NS2B, acts as the viral serine protease for polyprotein-processing. The NS1 glycoprotein plays a role in viral infection and pathogenesis, and sNS1 proteins can affect the complement pathway and protect DENV from complement-dependent neutralisation in solution. The role of other hydrophobic proteins like NS2A, NS4A, and NS4B is less well-characterized but may be involved in proper localisation of viral proteins and viral RNA during replication and virion assembly [17].
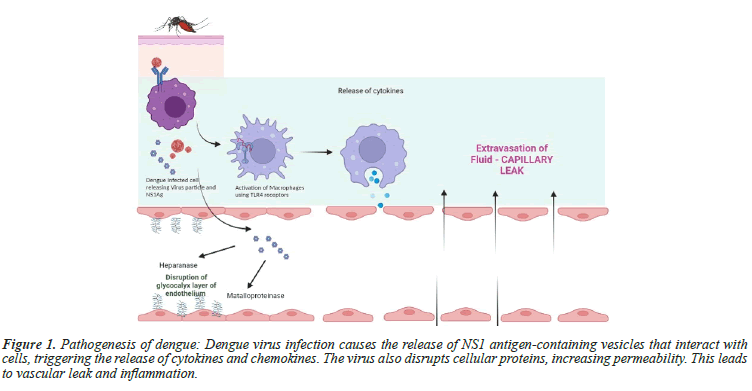
Vascular permeability: DENV infected cells produce extracellular vesicles containing NS1 antigen [18]. When monocytes, macrophages, and endothelial cells are exposed to NS1 antigen, they interact with Toll-Like Receptor 4 (TLR 4) on the cell surface, which triggers the release of various cytokines and chemokines like IL-1β and TNFα, inflammatory lipid mediators such as Platelet Activating Factor (PAF), which contribute to the vascular leak [19,20]. Helper innate T cells produce cytokines that mediate immune response or inflammation and correlate with disease severity [21]. DENV disrupts the cellular cytoskeletal proteins and lead to increased cellular permeability. The NS1 of the DENV virus can induce the necrosis of human endothelial cells by promoting the expression of Migration Inhibitory Factor (MIF). MIF, in turn, may increase the secretion of Heparanase-1 (HPA-1) and Matrix Metalloproteinase-9 (MMP-9), leading to the degradation of the endothelial glycocalyx and an increase in hyperpermeability [22]. Platelets also has been shown to increase vascular permeability through activation of NLRP-3 Inflammasome pathway, leading to secreation of IL-1 Beta [23]. The virus infected TH1 polarised mast cells also mediate cellular permeability [24]. Chemokines CXCL10, CXCL11, and their receptor CXCR3 have been linked to severe dengue infection and vascular permeability [25] (Figure 1).
Figure 1: Pathogenesis of dengue: Dengue virus infection causes the release of NS1 antigen-containing vesicles that interact with cells, triggering the release of cytokines and chemokines. The virus also disrupts cellular proteins, increasing permeability. This leads to vascular leak and inflammation.
Role of nitric oxide: Nitric Oxide (NO) levels are found to be increased in platelets of dengue patients, which cause vasodilatation and inhibition of platelet aggregation. Proinflammatory cytokine IL1β is also found to be elevated in dengue patients, which is known to induce NO synthesis in leukocytes. Its role in NO synthesis from platelets is yet to be elucidated [26]. Decreased serotonin levels in dengue patients also play role in capillary extravasation of fluid and thrombocytopenia. This can be a potential target for development of newer therapy [27].
Coagulopathy: Impaired hemostasis may manifests as self-limiting epistaxis and bleeding gums to lifethreatening gastrointestinal bleeding [28]. Coagulopathy in dengue can be caused by various factors, such as low platelets, abnormal PT, APTT, and hepatitis. Liver cell damage can decrease the synthesis of coagulation factors, affecting Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT). The NS1, has the ability to attach to both thrombin and prothrombin. When bound to thrombin, there are no observable effects. However, the activation of prothrombin is hindered. This phenomenon explains the early changes in APTT that are seen before the development of antibodies [29]. Furthermore, IL-6 can reduce the production of factor XII, which starts the intrinsic coagulation pathway [30].
Antibody Dependant Enhancement (ADE): Dengue virus infection has a unique pathophysiology where preinfection events may control the course of the infection. Experimental studies suggest that early acute-phase illness with higher peak viremia and antigenemia titres may lead to severe forms of dengue with a second infection [20]. Cross-reactive antibodies that bind to virus facilitate entry into host cells, followed by replication, thus increasing the cellular viral load. However, type-specific antibodies directed against the host-cell receptor binding domain of the dengue virus Envelope Domain-III (EDIII) exhibit a higher degree of specificity with lower potential of ADE [31]. Researchers have investigated the mechanisms behind ADE infection and suggest that DENV antagonizes the host antibody to enhance its fusion efficiency within macrophages, leading to the typical enhancement of infection, and stronger anti-inflammatory responses [32]. However, the contribution of afucosylated IgGs in dengueinduced ADE remains unclear [33].
Clinical manifestations
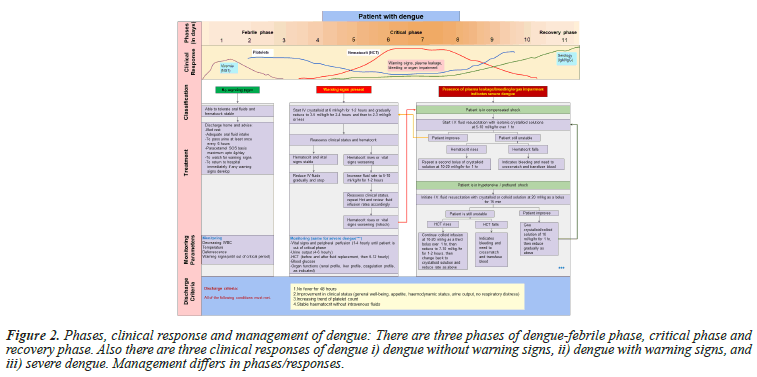
Clinical manifestations of dengue vary from self-limiting fever to Dengue Shock Syndrome (DSS). The mean incubation period is 5.9 days and 95% of cases developed symptoms between 3.4 and 10 days [34] (Figure 2).
Figure 2: Phases, clinical response and management of dengue: There are three phases of dengue-febrile phase, critical phase and recovery phase. Also there are three clinical responses of dengue i) dengue without warning signs, ii) dengue with warning signs, and iii) severe dengue. Management differs in phases/responses.
Dengue infection is seen in three phases
Febrile phase: The patients usually present with fever, myalgia, vomiting, headache, pain abdomen, back pain, and decreased appetite.
Critical phase: The critical phase of Dengue Fever (DF) is characterized by plasma leakage that can lead to shock. Signs of shock include coldness in the teguments, weak pulse, delayed capillary filling, tachycardia, oliguria, and hypotension. Patients with shock also exhibit signs of impaired haemostasis and gastrointestinal bleeding occurs in <10% of patients. Patients in this stage usually have a flushed face, a warm trunk, cold and clammy extremities, diaphoresis, slow venous filling, restlessness, irritability, pain in the upper and middle abdomen, and decreased urinary output. This phase lasts for 24-36 hours and is followed by rapid convalescence in those who recover.
Predictors of severe dengue: Not all patients develop the disease with similar severity. Multiple genetic factors have been associated with susceptibility to severe dengue infection. Single Nucleotide Polymorphisms (SNPs) in oligoadenylate synthetase genes, human leukocyte antigen class I and class II genes, tumour necrosis factor alpha, FcGRIIA, vitamin D receptor, transporters associated with antigen presentation, and Janus Kinase (JAK1) have been proposed. Genome-wide association studies have identified SNPs at the MICB and PLCE1 loci that increase the likelihood of developing dengue shock syndrome in children [35].
Younger age, presence of ascites, pleural effusion, low platelet counts and elevation of liver enzymes correlates with disease severity and likelihood of developing severe dengue [28,36]. Additionally, pre-existing conditions, such as diabetes, hypertension, renal disease, and cardiovascular disease, along with presenting symptoms like vomiting, abdominal pain and tenderness, bleeding, have also been associated with disease progression [16,37]. Severe dengue is more likely to occur during secondary infection when ADE is observed, leading to the development of DHF or vascular permeability syndrome. This finding was initially reported through epidemiological studies [38]. However, few recent studies have shown no differences in primary and secondary infection [39].
Renal manifestation in dengue: Renal involvement is a possible manifestation of dengue fever, presenting as Acute Kidney Injury (AKI), dyselectrolytemia, proteinuria, haematuria, and haemolytic uremic syndrome. Among hospitalized patients with severe dengue 3.3% to 4.8% develop AKI, of which 14.1% require renal replacement [40]. Hyponatremia and hypokalaemia are common electrolyte abnormalities in dengue patients. Mild hyponatremia is present in most dengue patients, and the cause may be due to excess water, inappropriate anti-diuretic hormone secretion, or a combination of factors. Hypokalaemia may be due to renal tubular abnormalities or redistribution of potassium within cells. Most patients in dengue presents with microscopic hematuria and proteinuria. Animal models and human infection have provided evidence that Glomerulonephritis (GN) in dengue is caused not only by immune complex deposition but also by direct viral entry into renal tissue. The reported cases have shown mesangial hypercellularity with immune complexes and IgM deposition, with some patients presenting with diffuse proliferative GN [41].
Ophthalmologic manifestations in dengue: Dengue fever is associated with ocular manifestations, with reported incidence ranging from 7.1% to 40.3%. The most common anterior segment manifestations include subconjunctival haemorrhages and anterior uveitis, which can present with various symptoms. Rare anterior segment conditions, such as acute angle-closure glaucoma, have also been reported. Posterior segment manifestations are less common but can have more significant consequences, with maculopathy being the most common, reported in about 10% of hospitalized patients [42].
Dengue can cause persistent myalgia and arthralgia beyond the acute phase and can also lead to myocarditis in some cases. Anatomopathological studies show perivascular mononuclear infiltrates and fibrosis in DENV-infected patients with arboviral-induced myositis, and inflammatory markers and DENV antigens are found in myocardial cells in cases of DENV-induced myocarditis [43].
Cutaneous findings are a prominent feature of DF and Dengue Hemorrhagic Fever (DHF). DF is characterized by two rashes: The initial flushing erythema of the face and a second maculopapular or morbilliform rash that occurs 3-6 days after fever onset. This rash is typically asymptomatic and lasts several days, starting on the hands and feet and spreading to the torso. Less frequently, other types of rashes may occur. Haemorrhagic manifestations such as petechiae, purpura, or ecchymosis with a positive tourniquet test are common in DHF and DSS and rarely in DF [44].
Rare manifestations of dengue: A rare and potentially fatal condition called Hemophagocytic Lymphohistiocytosis (HLH) is increasingly being recognized in severe cases of dengue. HLH is characterized by fever, low levels of blood cells, enlarged liver and spleen, and high levels of serum ferritin [45]. HLH cases have also been reported in Asia, with many occurring after prolonged fever. An early diagnosis of HLH could prevent misdiagnosis and inappropriate treatment for vascular permeability shock syndrome with steroids, which have been ineffective [46].
Diffuse Alveolar Haemorrhage (DAH), which is characterized by haemoptysis, pulmonary infiltrates on chest X-ray, and anaemia, can occur without haemoptysis in around 40% of cases. DAH in dengue haemorrhagic fever being a rare occurrence that is scarcely reported in the literature [47].
Dengue and pregnancy: Dengue infection poses a higher risk of severe complications in pregnant women, with maternal mortality estimated to be increased by a factor of three. Diagnosis may be difficult due to overlap with pregnancy-specific diseases such as preeclampsia and gestational thrombocytopenia, and physiological haemodilution may delay diagnosis. Complications in pregnant women may include hypovolemic shock, haemorrhages, and preeclampsia. Severe dengue infection was associated with an increased risk of post-partum haemorrhage. Maternal-fetal transmission of dengue has been documented, with up to 56.2% risk in infections within 15 days prior to and 2 days after delivery. The timing of delivery should be decided by experienced obstetricians based on maternal status, disease severity, fetal well-being, and gestational age. There is no increased risk of malformations in newborns of pregnant women with symptomatic dengue infection, but significant heterogeneities exist between studies [48,49].
Dengue in solid organ transplant patients: Dengue infection in Solid Organ Transplant (SOT) recipients can result in Severe Disease (SD) and impaired graft function, but early diagnosis and access to intensive care units can improve outcomes. Some cases required adjustments in immunosuppressive treatment, but this was not related to dengue severity or mortality. Secondary dengue infection is associated with a higher risk of SD, and differentiating primary from secondary dengue can be difficult in SOT. Dengue diagnosis among SOT recipients can be challenging due to comorbidities and adverse effects of immunosuppressive drugs. In some cases, dengue transmission can occur from donors to recipients, and donor screening should be considered in endemic areas. Dengue in the first month after transplantation may constitute a severe condition, and SOT recipients may have less fever and arthralgia due to immunosuppressive therapy. Elevated serum creatinine and acute renal failure may occur in kidney recipients [50].
Diagnosis
The WHO 1997 classification for dengue is a strict longitudinal classification that relies on specific clinical and biological signs to determine the severity of the disease. In contrast, the WHO 2009 classification is a more flexible transversal classification that can be used with ultrasound to accurately identify severe cases of dengue for appropriate clinical management of hospitalized patients [51]. However, there are concerns that the recommendation to admit all patients with dengue with warning signs may increase the total volume of admissions and adversely affect the quality of care given to hospitalized patients. While no quantitative studies have assessed the use of the 2009 D/SD (Dengue/Severe Dengue) classification versus the 1997 DF/DHF/DSS (Dengue Fever/ Dengue Haemorrhagic Fever/ Dengue Shock syndrome) classification for surveillance purposes, the difficulties in applying DF/DHF/DSS led to the development of multiple local adaptations and put global comparability at risk. Furthermore, the level of evidence for most Warning Signs (WS) established in D/SD is not high, and large quantitative studies are needed to establish their validity. There is currently no quantitative evidence on the use of D/SD in dengue surveillance and case reporting [52].
Torniquest test: The tourniquet test, which measures capillary fragility, is included in the WHO guidelines as a diagnostic sign for dengue. However, a meta-analysis of 16 studies found poor diagnostic performance with low sensitivity and specificity. A retrospective analysis of >28000 tourniquet tests also found no association between test results and final laboratory-confirmed diagnosis or dengue severity. The poor biological correlation between dengue infection and capillary fragility may be underlying the test’s poor diagnostic performance (sensitivity: 58% and specificity: 71%), along with practical considerations such as difficulty of interpretation in different skin colours and uncertainties about its positivity in other flavivirus infections. Therefore, it may be time to stop using the tourniquet test as a diagnostic criterion for dengue.
Serology: Identification of viremia is possible as early as 24-48 hours prior to the onset of fever and lasts for around 5-6 days. During this time, the virus, as well as its specific RNA and NS1 protein, can be detected in patient blood, serum, plasma, and tissues from fatal cases. Various methods available are discussed here.
Antibody serology: Measuring anti-dengue Immunoglobulin M (IgM) and/or IgG antibodies using an Enzyme Linked Immunosorbent Assay (ELISA) test is the most widely used method for diagnosing dengue. However, the sensitivity of IgM ELISAs is limited to less than 50% during the first 4 days after symptoms appear in primary infections, making them less useful for clinical management. On the other hand, the IgG titre is higher in secondary infections, allowing the IgM:IgG ratio to differentiate primary from secondary infections during the acute phase of the disease. While some laboratories and commercial vendors use a fixed cut-off of 1.2-1.4, this varies between facilities. A recent study suggested that using a dynamic cut-off strategy based on the number of days after symptom onset led to better results. This study established a range of cut-offs for defining primary infections using the IgM:IgG ratio, ranging from 1.8 on day 2 to 1.0 on day 7, with a sensitivity of 90%, specificity of 77%, and accuracy of 84% [53].
NS1 antigen: NS1 is a glycoprotein secreted as a hexamer during dengue infection and can be detected in the acute phase of the disease. Unlike IgM and IgG, NS1 is detectable during the acute viraemic phase of infection, which is when the virus is actively replicating and is specific to dengue. Its early presence and abundance in serum, make it ideal for early diagnosis, with many commercial tests available or in development. Rapid diagnostic Tests using NS1 can swiftly detect imported cases of dengue at airports to prevent its spread during epidemics. ELISA detection methods are also available [53].
Nucleic acid detection: The Reverse Transcription Polymerase Chain Reaction (RT-PCR) is a fast, sensitive and specific diagnostic test for detecting viral RNA, but it has limitations such as a short window of opportunity and high risk of contamination. Various RT-PCR techniques have been developed, including real-time RT-PCR, which is faster and less prone to contamination. Low-cost alternatives to RT-PCR, such as hydrolysis-based assays, are being developed to improve dengue diagnostics in low-resource settings. However, a lack of standardisation in PCR methods across laboratories makes it difficult to compare results. This variation is a barrier for improving dengue diagnostics, and there is a need for standardisation in PCR methods [53].
The IMA (Immuno Magnetometric Assay) test uses magnetic nanoparticles coated with monoclonal antibodies to detect NS1 from all four DENV serotypes. The detection mechanism relies on the aggregation of magnetic nanoparticles that can be read electronically. A study analyzed 135 serum samples from travelers returning from dengue-endemic countries and compared the IMA test with other rapid tests, including the ICT type and ELISA. The IMA test had a better sensitivity compared to ICT however it’s sensitivity was low [54].
The availability of USG is limited in resource-constrained areas, so a Dengue Score system has been developed to predict pleural effusion and/or ascites using laboratory parameters. The score is based on several parameters such as degree of hemoconcentration, lowest albumin concentration, degree of hypoalbuminemia, lowest platelet count, elevated AST, ALT and sodium concentrations. The score can identify patients at risk of developing severe dengue and has a sensitivity of 82.47%, specificity of 70.42%, PPV of 79.21%, NPV of 74.63%, and a correct prediction rate of 77.38% at a cut off of ≥ 2 [55].
A new immunosensor for detecting the dengue virus using hybrid nanomaterial has been developed. The sensor consists of modified maghemite nanoparticles bound to gold nanoparticles conjugated with aptamers, creating a colorimetric immunosensor. Specific aptamers for the four dengue serotypes were modified to bind to the nanoparticles, and the solution changes colour upon detecting the virus. The method is easy, fast, low-cost, and specific, making it suitable for field measurements for the diagnosis of dengue [54].
Blood, plasma, and serum are commonly used specimens for diagnosing dengue infection, but studies have shown that saliva and urine can also be used as non-invasive alternatives. The high sensitivity (93.3%), specificity (100%), positive predictive value (100%), and negative predictive value (83.3%) of detecting IgG in saliva make it a promising sample type for diagnosing dengue fever. DENV RNA has been detected in both saliva and urine, and urine can also be used to detect NS1 antigen. However, the sensitivity of urine and saliva samples for detecting DENV is lower than that of plasma samples. Further research is needed to optimize the use of urine and saliva for dengue diagnostics, but their potential as alternative samples is promising, especially in resourcelimited settings [56] (Table 2).
| Clinical sample | Diagnostic method | Methodology | Time to results | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| Virus detection and its components | Acute serum (1-5 days of fever) and necropsy tissues |
Viral isolation | Mosquito or mosquito cell culture inoculation | One week or more | - | - |
| Nucleic acid detection | RT-PCR and real time RT-PCR | 1 or 2 days | 83.9-90.3 [57] | 100 [57] | ||
| Antigen detection | NS1 Ag rapid tests | Minutes | 55-82 [58] | 97-100 [58] | ||
| NS1 Ag ELISA | 1 day | 89.9 [59] | 100 [59] | |||
| lmmuno-histochemistry | 2-5 days | - | - | |||
| Serological response | Paired sera (acute serum from 1-5 days and second serum 15-21 days after) | lgM or lgG seroconversion | ELISA HIA |
1-2 days | - | - |
| Neutralization test | Minimum 7 days | - | - | |||
| Serum after day 5 of fever | lgM detection (recent infection) | ELISA | 1 or 2 days | 79.840 | 20.140 | |
| Rapid tests | Minutes | 59.740 | 50.240 | |||
| lgG detection | lgG ELISA HIA |
1 or 2 days | 59.740 | 40.240 |
Note: ELISA: Enzyme-linked Immunosorbent Assay; HIA: Haemagglutination Inhibition Assay; IgG: Immunoglobulin G;
IgM: Immunoglobulin M; NS1 Ag: Non-Structural protein 1 Antigen; RT-PCR: Reverse Transcriptase Polymerase Chain Reaction.
Table 2. Dengue diagnostics and sample characteristics.
A study found that PCT (Procalcitonin) levels ≥ 0.7 ng/ mL was independently associated with dengue shock and/ or organ failure. However concurrent bacterial infections were observed in 4-25% of adults with dengue after median duration of 6.5 days, and were more common among those with severe plasma leakage which could also increase procalcitonin levels [60].
Atypical Lymphocytes (AL) with increased nucleic acid percentage and characteristic scatter properties can be detected by an increase in fluorescence signal during Dengue virus Infection (DI) progression. These AL have been identified as CD19+ B lymphocytes and are thought to be an immune reaction to the dengue virus, explaining the significant increase in anti-dengue Immunoglobulin G (IgG) antibodies during the secondary dengue infection. Recently, atypical plasmacytoid lymphocytes have also been observed in patients with dengue infection, correlating with elevated IgG levels. AL has been shown to be useful for differentiating DI from other viral infections. The association of AL with the severity of dengue infection is currently being assessed [61].
Dengue infection causes the enhanced secretion of multiple cytokines by innate immune cells, with TNF-α dominating in CD56+, CD3+, NKT cells, monocyte subsets, and granulocytes, along with IFN-γ from CD56+, CD3+, NKT cells. Higher proportions of TNF-α-secreting granulocytes and monocyte subsets were associated with mild dengue and minimal symptoms. Dengue NS1 antigenemia, used as a surrogate of viral load, directly correlated with the proportion of cytokine-secreting innate immune cells, and was significantly higher in patients who recovered with minimal symptoms. However, in patients with secondary dengue or those with bleeding or elevated liver enzymes, early activation as well as efficient downregulation of innate responses were compromised, which revealed a predisposition to severe outcomes [62].
Treatment
Treatment of dengue depends on the clinical condition of the patient. Those presenting early without any warning signs can be treated on an outpatient basis with acetaminophen and adequate oral fluids. Such patients should be explained regarding the danger signs and asked to report to hospital immediately if they notice any of them. Those patients with warning signs, severe dengue or other situations like infancy, elderly, pregnancy, diabetes and those living alone need to be admitted.
Those with warning signs can be initiated on IV crystalloids and fluid rate is titrated based on the patient's response as given in Figure 2. Colloids can be started for patients in shock and are also preferred if the patient has already received previous boluses of crystalloid and has not responded [63]. Blood transfusion is warranted in case of severe bleeding or suspected bleeding when patient remains unstable and hematocrit falls despite adequate fluid resuscitation [64]. Platelet transfusion is considered when platelet count <20,000/cumm and there is high risk of bleeding. Patients with platelets in the range of 21,000 to 40,000 require transfusion if they have bleeding manifestations [64].
The maintenance fluid required for an individual depends on their caloric expenditure, and the 100/50/20 rule developed by Holliday is widely used to calculate the volume of maintenance fluid required. A 5% deficit of the maintenance fluid is added to calculate the fluid quota during the 48 hours of the critical phase. Intravenous crystalloid solutions or colloids such as dextran-40 in saline are recommended for fluid management, and blood transfusion is recommended if bleeding is suspected during fluid resuscitation. Some patients may require fluid in excess of the currently recommended Maintenance +5% deficit to maintain organ perfusion, and this group of patients tend to have a higher Body Mass Index (BMI), lower white cell count at the onset of the critical phase, and more severe disease compared to patients requiring fluid within the recommended quota. The amount of fluid required should be adjusted according to the urine output, clinical parameters, and the Packed Cell Volume (PCV), and a stepwise pattern should be followed during the critical period [65].
Several studies that have observed the efficacy of prophylactic platelet transfusion in preventing bleeding in adults with dengue and thrombocytopenia [66].
Numerous studies have investigated the efficacy of corticosteroid treatment for dengue shock syndrome. The results have been mixed, with some studies showing a significant reduction in mortality risk with corticosteroids, while others have found no benefit in terms of pulmonary hemorrhage, seizures, and length of hospital stay [67]. A study investigated the effect of prednisolone on the host immune response during dengue infection and found that high-dose prednisolone therapy was associated with underabundance of transcripts encoding T and NK effector proteins, which may suggest impaired antiviral cytolytic responses. However, transcripts from neutrophils were more abundant in high-dose prednisolone treated patients, and further studies are needed to understand the functional neutrophil response [68].
Novel therapies in dengue: NS2B/NS3 protease is a DENV enzyme that helps in replication and assembly of the virus. Antiviral drugs targeting this enzyme are being developed [69].
Rupatadine, a PAF inhibitor, is found to reduce the incidence of dengue hemorrhagic fever when administered to patients with dengue [19].
Human Monoclonal Antibodies (HuMAbs), which act against NS1 antibodies, have been isolated from patients infected with dengue. However, their usage was limited due to cross reactivity with plasminogen [70]. Human monoclonal antibody, IG5, has been found to neutralise DENV1 and is to be studied as a prophylactic and therapeutic option [71]. Many antiflavivirals like protease inhibitors, NS5 polymerase inhibitors, entry/fusion inhibitors and helicase inhibitors are in developmental phase [72].
Antiflavivirals: They are
1. Peptide and non-peptide NS3/N2SB Protease inhibitors
2. NS3 helicase inhibitors 3. Entry inhibitors 4. RdRp inhibitors 5. MTase inhibitors 6. Inhibitors targeting host protein like lactidomycin, sacratinib and dasatinib
Plasmid based gene modification has found to produce DENV neutralising antibodies in animal models and a possible therapeutic option in future [73].
Several plants have been investigated for their potential to treat dengue fever. C. papaya leaf has shown potential by increasing platelet count, white blood cells, and neutrophils in a patient bitten by carrier mosquitoes. Cymbopogon citratus and Euphorbia hirta have also been studied, but their antiviral activity against dengue was found to be limited. Piper retrofractum showed inhibitory activity against DENV-2 infected cells. Essential oils from Lippia alba and Lippia citriodora have shown considerable inhibitory effects on dengue virus serotype replication.
Coinfection
Dengue and SARS-CoV 2: Fever is a common symptom of both dengue and COVID-19, which makes it difficult to differentiate between the two infections. However, the presence of additional symptoms such as breathing difficulties, cough, headache, and loss of taste may help in distinguishing COVID-19. In the case of co-infection, COVID-19 may not be considered as a possible diagnosis if there are no respiratory symptoms, but testing should be performed if the patient does not respond to dengue treatment. Due to the overlapping symptoms of the two diseases, healthcare providers should consider both as potential diagnoses in patients with fever and other non-specific symptoms.
SARS-CoV-2 and dengue virus co-infection often present with thrombocytopenia, likely due to virus-induced bone marrow suppression and immune-mediated clearance of platelets. Autoantibodies and immune complexes produced in response to the viruses may also destroy platelets. Patients with comorbidities, particularly diabetes, hypertension, and cardiovascular disease, are more likely to result in severe illness and higher case fatalities in both diseases [74].
Malaria: Malaria is a common co-infection with dengue fever, especially in endemic regions like India. Excluding malaria diagnosis early is important as its treatment requires different medication. Complicated vivax malaria is on the rise, and co-infection should be suspected in patients with jaundice or spontaneous bleeding. Testing with a rapid diagnostic test kit for malaria should be done at the first presentation. To differentiate between dengue and malaria infections, specific markers such as atypical lymphocytosis, hemoconcentration, and thrombocytopenia can be useful, as they are indicative of dengue infection. Patients with co-infection and those with Plasmodium mono-infection were found to be at a greater risk of developing severe dengue. Among the patients with severe dengue with malaria coinfection, almost half experienced severe bleeding/bleeding, while jaundice was observed in more than 30% of the patients. Early antimalarial treatment can prevent complications and improve outcomes [75,76] (Table 3).
| No. | Coinfection | Transmission | Overlapping symptoms and signs | Distinguishing symptoms and signs of Dengue | Distinguishing symptoms and signs of Coinfection | Laboratory confirmation of Coinfection | Distinguishing prevention and treatment for Coinfection | When to suspect Coinfection | Prognosis of Coinfection | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Malaria | Anopheles mosquito bite | Fever, fatigue, malaise, thrombocytopenia | Bleeding manifestations, hemoconcentration, and thrombocytopenia | Fever with typical paroxysm, cerebral Malaria, renal failure | Microscopy Rapid diagnostic tests to detect malarial antigen |
Chloroquine, Artemisinin-based combination therapy. Insecticide-treated nets, indoor residual sprays and larval source management reduce mosquito density. |
Endemic areas. Lower AST, ALT levels, higher platelet count and hemoglobin |
Increased risk of severe Malaria in Coinfection. | [77-79] |
| 2 | Chikungunya | Aedes albopictus mosquito bite | Fever, headache, myalgia | Bleeding manifestations, pain abdomen | Arthralgia, joint swelling, predominant. Shorter duration of fever | Demonstration of IgM and IgG antiCHIKV antibodies using ELISA or indirect immunofluorescence. Reverse transcriptase PCR or real time PCR. |
Supportive care with rest, hydration, and analgesic agents. Chronic manifestations can be treated with steroids and Disease-Modifying Anti-Rheumatic Drugs (DMARDs). Using mosquito repellents, wearing long clothes with long sleeves, and indoor and outdoor mosquito control measures |
Endemic areas. | More morbidity and mortality | [80-82] |
| 3 | Yellow fever | Aedes aegypti mosquito bite | Fever, myalgia, arthralgia, rash and headache | Similar features | Predominant jaundice | Rapid tests. Demonstration of antibodies by ELISA |
Yellow fever vaccine (live-attenuated 17D virus). Mosquito control measures |
Cocirculation of arboviruses. Travelers from endemic areas. |
Variable | [83,84] |
| 4 | COVID 19 | Direct, Indirect or close contact with respiratory secretions of an infected person | Fever, fatigue, myalgia | Bleeding manifestations | Shortness of breath, cough | Real-time PCR, SARS CoV 2 antigen tests |
Supportive treatment with oxygen, Remdesivir, Dexamethasone, and Tocilizumab. Hand hygiene, face mask and social distancing is the key to prevention. Vaccination is highly effective. |
Contact with patient infected with covid 19 | Increased morbidity due to misdiagnosis | [85,86] |
| 5 | Hantavirus | Inhalation of aerosols from feces, urine or saliva of rodents hosting the virus | Fever, myalgia, headache | Bleeding manifestations, thrombocytopenia | Respiratory distress, cough (hantavirus pulmonary syndrome) | Anti–hantavirus IgM and IgG ELISA | Supportive measures for Pulmonary syndrome | Endemic areas (stagnant water and mosquitoes, rodent invasion). Exposure to wild rodents for 1–6 weeks before symptom onset |
May increase in mortality. A good prognosis is also reported | [87] |
| 6 | Hepatitis A | Feco oral route | Fever, headache, fatigue, myalgia, and abdominal discomfort | Thrombocytopenia, hemoconcentration, third space fluid loss | Icterus, high coloured urine, marked increase in serum aminotransferase levels, deranged prothrombin time | Hepatitis A IgM antibodies test using enzyme-linked immunosorbent assay | Supportive. Hygienic food practices. |
During outbreaks and when a patient presents with jaundice | Usually self-limited | [88] |
| 7 | Zika virus | Aedes aegypti and Aedes albopictus mosquito bite | Fever, myalgia | Hemorrhagic fever | Conjunctivitis, early diffuse skin rash with short-lasting fever, and joint edema may be seen in a few cases | Zika virus RTPCR | Rest, adequate hydration, antipyretics and analgesics. Mosquito control measures. | In endemic areas, a patient presenting with fever and diffuse pruritic maculopapular rash | It can be less severe with good clinical outcome | [89-91] |
| 8 | HIV | Sexual contact, maternal-infant exposure, blood product transfusion and by percutaneous inoculation | Fever, pain abdomen. | Acute presentation with fever, headache, pain abdomen, hemorrhagic manifestations | Prolonged history of fever, weight loss, night sweats, chronic diarrhea | Rapid tests, Western blot tests, PCR, 4th generation antigen or antibody tests | Highly Active Antiretroviral Therapy (HAART) | In a patient with high-risk behaviour, chronic diarrhea, weight loss. | No severe complications. Reduction in HIV1 viral load |
[92-94] |
| 9 | Typhoid | Feco oral transmission | Fever, headache, arthralgia | Retro orbital headache, hemorrhagic manifestations | saddleback fever (there are spikes in temperature without any return to normal), Constipation or diarrhea | Widal test and ELISA to detect antibodies. Culture from blood, stool, and bone marrow (gold standard) | Antibiotic therapy (fluoroquinolones, Azithromycin, cephalosporins, cotrimoxazole). Vaccination - Vi capsular polysaccharide vaccine, Ty21a vaccine. Hygienic food practices. |
During outbreaks, particularly during Monsoon | Increased complications | [95,96] |
Table 3. Literature review of clinical profile of dengue co-infections.
Prevention and control of dengue infection
Preventive measures are of utmost importance especially in the endemic areas like South Asia where optimal temperature favours breeding of Aedes mosquito [97]. The preventive strategies have been elaborated under following headings
• Community based control programs: These programs aim to control and exterminate the breeding sites of the vector by educating the community members regarding the vector and disease [98]. Conducting awareness programs like mass campaigns among different community groups and audio visual programs on regular time intervals especially targeting the breeding season are effective.
• Vector control: Vector control is the most important part of any vector borne disease control program. Major approaches of mosquito control to prevent DENV infection are discussed as follows
• Biological control
Biological control can be done as per follows
Fish: Predatory larvivores fish have been found effective to reduce the burden of mosquito larvae in the container. Among the different species, Gambusia affinis and Poecilia reticulate are the most important one, with the first being resistant to commonly used insecticide too [99].
Endosymbiotic control: Releasing mosquitoes infected with Wolbachia (an intracellular bacterium) makes them less susceptible to the Dengue infection as compared to the wild-Aedes mosquito [100].This bacterium competes with the virus for cholesterol and iron making it harder for the virus to multiply [101].
• Predatory copepods: Seeding water vessels with fresh copepods which are small fresh water crustaceans have been found successful especially in rural communities.
• In addition to this, mosquitocidal fungi have also been found effective in some studies.
• Chemical control: It includes use of larvicides in breeding containers and insecticide spraying. Space sprays preferably oil based formulation have been applied as thermal fogs or cold aerosols. Compounds most commonly used include pyriproxyfen, d-tetramethrin metofluthrin, and cyphenothrin [102,103].
• Environmental measures: Covering containers with insecticides, waster management without direct garbage collection and elimination of breeding places are simple but effective environemntal measures for vector control [104]. Community mobilization can add effectiveness to the vector control program with added advantage of local customization and strong community participation [105].
• Personal protective measures: Use of mosquito repellents (containing DEET, IR3535, or icaridin), wearing full sleeve clothes, long-lasting insecticide treated bed nets (deltamethrin, alphacypermethrin) and mosquito traps using carbon dioxide producer are some measures for personal protection.
• Vaccination: For the last two decades , efforts have been made to develop safe and effective vaccines to prevent Dengue infection [106]. Different dengue Barbosavaccines that have reached phase III or have been licensed are tabulated as follows (Table 4).
| Vaccine | Type of vaccine | Dosing schedule | Efficacy | Comments | References |
|---|---|---|---|---|---|
| Licensed | |||||
| CYT-TDV/Dengvaxia | Live recombinant tetravalent vaccine | 3 doses (0/6/12 months) | 25-59% | Hospitalization increased in seronegative vaccinees | [107] |
| Phase III | |||||
| TAK-003/DENVax | Tetravalent attenuated vaccine | 2 doses (0/3 months) | 73.5-85.3% | Better tolerance | [108] |
| LATV (TV003/TV005) | Live attenuared tetravalent vaccine | Single dose | Not yet released | Single dose | [109] |
Table 4. Dengue vaccines.
In addition to these, other five vaccines are in phase I and phase II clinical trials [110,111].
Discussion and Conclusion
Concerned with the treatment implications, drugs targeting the flavivirus proteases mainly the NS2-NS3 active site have been developed that could be effective in the treatement of dengue fever. These active sites bear similarity with other flaviviurses like Zika virus and West Nile virus. Direct viral inhibitors and modifiers of virushost interaction have shown to reduce viremia and levels of proinflammatory cytokines in the dengue mouse model. As stated earlier, there are seven dengue vaccines that are under different phases of clinical trials. Several research mysteries still remain: Why is the onset of capillary permeability correlated with defervescence period, why do cross infection by different serotypes produce greater severity and what are the crucial components of protective immunity against dengue?
References
- Zeng Z, Zhan J, Chen L, Chen H, Cheng S. Global, regional and national dengue burden from 1990 to 2017: A systematic analysis based on the global burden of disease study 2017. EClinicalMedicine 2021; 32: 100712.
[Google Scholar] [PubMed] [Crossref]
- Wilder-Smith A, Rupali P. Estimating the dengue burden in India. Lancet Glob Health 2019; 7: e988-e989.
- Mustafa MS, Rasotgi V, Jain S, Gupta V. Discovery of fifth serotype of Dengue Virus (DENV-5): A new public health dilemma in dengue control. Med J Armed Forces India 2015; 71: 67-70.
[Google Scholar] [PubMed] [Crossref]
- Murhekar MV, Kamaraj P, Kumar MS, Khan SA, Allam RR, Barde P. Burden of dengue infection in India, 2017: A cross-sectional population based serosurvey. Lancet Glob Health 2019; 7: e1065-e1073.
[Google Scholar] [PubMed] [Crossref]
- Uno N, Ross TM. Dengue virus and the host innate immune response. Emerg Microbes Infect 2018; 7: 167.
[Google Scholar] [PubMed] [Crossref]
- Roy SK, Bhattacharjee S. Dengue virus: Epidemiology, biology, and disease aetiology. Can J Microbiol 2021; 67: 687-702.
[Google Scholar] [PubMed] [Crossref]
- Sylvestre E, Joachim C, Cécilia-Joseph E, Bouzillé G, Campillo-Gimenez B, Cuggia M. Data-driven methods for dengue prediction and surveillance using real-world and Big Data: A systematic review. PLoS Negl Trop Dis 2022; 16: e0010056.
[Google Scholar] [PubMed] [Crossref]
- Horta MA, Bruniera R, Ker F, Catita C, Ferreira AP. Temporal relationship between environmental factors and the occurrence of dengue fever. Int J Environ Health Res 2014; 24: 471-481.
[Google Scholar] [PubMed] [Crossref]