Research Article - Biomedical Research (2017) Volume 28, Issue 13
Decreased expression of miR-495 is associated with poor prognosis in clear cell renal cell carcinoma
Zheng Shubei, Zheng Yu, Jin lingwei, Zhou Zhihong and Li Zhanyuan*
Department of Nephrology, the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, PR China
- *Corresponding Author:
- Li Zhanyuan
Department of Nephrology
The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, PR China
Accepted on May 26, 2017
Abstract
Objective: The purpose of this study was to investigate whether miR-495 is a factor influencing survival in clear cell renal cell carcinoma (ccRCC) patients.
Methods: Quantitative real-time PCR (qRTPCR) was performed to evaluate the expression level of miR-495 in 186 participants. Then the association between tissue miR-495 expression level and clinical outcome was investigated. Overall survival was evaluated using the Kaplan-Meier method. Multivariate analysis of the prognostic factors was performed with Cox proportional hazards model.
Results: The expression level of miR-495 was significantly decreased in renal cancer tissues compared with that in normal matched tissues, and a high expression of miR-495 was found to be significantly associated with Histologic grade (p=0.000), Lymph nodemetastasis (p=0.000), and Distant metastasis (p<0.001). In addition, the results of Log-rank test indicated that ccRCC patients with low miR-495 expression experienced shorter overall survival. Multivariate analysis suggested that miR-495 expression was an independent prognostic factor for overall survival of patients with ccRCC.
Conclusions: Our results indicate that miR-495 relates to the prognosis of patients with ccRCC and may act as a promising predictor of ccRCC recurrence.
Keywords
MiR-495, Clear cell renal cell carcinoma, Quantitative real-time PCR, Prognosis.
Introduction
Renal cell carcinoma (RCC) is responsible for approximately 3% of all cancers in adults and is the third most common urological cancer, with incidence rates increasing 2% per year [1,2]. Approximately 60,920 novel cases of RCC were diagnosed in the United States in 2011, with an estimated 13,120 mortalities [3]. Clear cell RCC (ccRCC) is the most type of RCC. Radical nephrectomy is effective to cure early and local ccRCC, but patients with metastatic RCC [4] face a poor prognosis and have limited therapeutic options. Therefore, investigating the pathogenesis and biological features of ccRCC is crucial to enhance early detection and treatment.
MicroRNAs (miRNAs) are short non-coding regulatory RNA involved in regulation of important cellular processes as differentiation, proliferation, cell cycle, or apoptosis [5,6]. Accumulating evidence has shown that miRNAs can participate in tumour genesis, progression and metastasis either as oncogenes or tumour suppressors [7,8]. MiR-495 was reported to act as a tumor suppressor gene or an oncogene in a lot of cancers including non-small cell lung cancer, breast cancer, glioblastoma, and gastric cancer [9-11]. Recently, Lv et al. found that miR-495 suppresses human renal cell carcinoma malignancy by targeting SATB1 [12]. Those results informed that down-regulation of miR-495 levels are associated with worse outcome in ccRCC. However, to our knowledge, the relationship between the expression of miR-495 and survival in ccRCC patients remains to be determined.
In the present study, we explored the expression of miR-495 in human ccRCC tissues and investigated the correlation between the expression of miR-495 and clinicopathologic factors and survival in ccRCC patients.
Materials and Methods
Patients and tissue samples
This study was approved by the Institutional Review Board of the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University. All patients written informed consent and are agreed to participate in this study. All samples were obtained with informed consent and approved by the hospital institutional review board.
Fresh clinical ccRCC specimens and adjacent normal tissues were collected from 166 patients who underwent radical nephrectomy between 2007 and 2010 in the Second Affiliated Hospital & Yuying Children’s Hospital of Wenzhou Medical University. All patients did not receive anticancer treatment, including chemotherapy, radiotherapy and biotherapy, prior to surgery resection. Samples were flash frozen in liquid nitrogen until use. The clinical and pathological information from patient records was gathered, and the details were listed in Table 1.
| Variables | Cases (n=166) | miR-495 expression level | p value | |
|---|---|---|---|---|
| High expression | Low expression | |||
| Age (years) | 0.660 | |||
| <55 | 106 | 51 | 55 | |
| ≥ 55 | 60 | 31 | 29 | |
| Gender | 0.762 | |||
| man | 79 | 40 | 39 | |
| woman | 87 | 42 | 45 | |
| Tumor size (cm) | 0.750 | |||
| <4 | 79 | 38 | 41 | |
| ≥ 4 | 87 | 44 | 43 | |
| Histologic grade | 0.000 | |||
| I-II | 94 | 32 | 62 | |
| III-IV | 72 | 50 | 22 | |
| Tumor stage | 0.218 | |||
| T1-T2 | 75 | 41 | 34 | |
| T3-T4 | 91 | 41 | 50 | |
| Lymph nodemetastasis | 0.000 | |||
| Absence | 65 | 45 | 20 | |
| Presence | 101 | 37 | 64 | |
| Distant metastasis | 0.001 | |||
| Absence | 66 | 43 | 23 | |
| Presence | 100 | 39 | 61 | |
Table 1. Clinicopathological features and miR-495 expression in ccRCCpatients.
qRT-PCR of miR-495 expression
Total RNA was extracted from frozen tissue using TRIzol Reagent (Applied Invitrogen, Carlsbad, CA, USA). RNA concentration and purification were conducted using the NanoDrop ND-1000 spectrophotometer (Nanodrop, Wilmington, DE, USA). The expression level of miR-495 was analyzed using the Hairpin-it miRNA qPCR.
Quantitation Kit (GenePharma, Shanghai, China) following the manufacturer’s instructions. Reverse transcription and quantitative PCR were performed using the One Step PrimeScript miRNA cDNA Synthesis Kit (Takara, Dalian, China) by using the ABI 7500 Real Time PCR system (Applied Biosystems, Foster City, CA, USA). Relative quantification of target miRNA expression was evaluated using the comparative cycle threshold (CT) method. U6small nuclear RNA was used as an internal control.
Statistical analysis
Statistical analysis was conducted using the SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA). The distinct expression of miR-495 between tumor tissues and normal tissues was examined by independent samples t-test. Chis-quare test and t test were performed to explore the associations between miR-495 expression and clinical characteristics. The overall survival was analyzed by log-rank test, and survival curve was plotted based on Kaplan-Meier method. Multivariate analysis was performed using the Cox proportional hazard model. P values less than 0.05 were considered statistically significant.
Results
MiR-495 expression decreases in human ccRCC
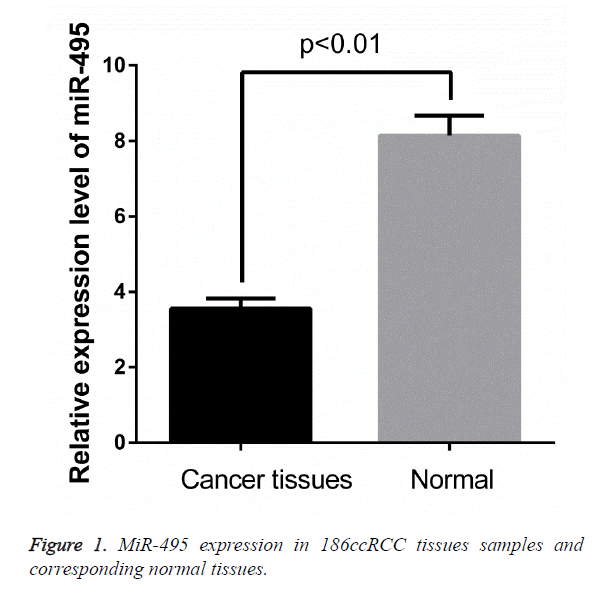
To investigate the potential roles of miR-495 in ccRCC development, The expression levels of miR-495 in osteosarcoma and corresponding noncancerous biopsy samples were detected by qRT-PCR and normalized to U6. The results showed that ccRCC tissues had significantly lower miR-495 expression levels (P<0.01; Figures 1 and 2) compared to noncancerous tissues.
Association of miR-495 expression with the linicopathological characteristics of human ccRCC
The correlation of miR-495 expression with different clinicopathological parameters in gliomas was illustrated in Table 2. Low expression of miR-495 was found to significantly correlate with higher histological grade (p=0.000), lymph node metastasis (p=0.000) and tumor distant metastasis (p=0.001). No significant correlations were observed between miR-495 expression and any other clinicopathological features, such as gender, age, tumor size, tumor stage (p>0.05).
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Risk ratio | 95% CI | p | Risk ratio | 95% CI | p | |
| Gender Male vs. Female |
1.412 | 0.771-1.498 | 0.617 | |||
| Age (years) ≥ 55 vs. <55 |
1.332 | 0.417-1.659 | 0.326 | |||
| Tumor stage T3-4 vs. T1-2 |
2.441 | 1.983-3.771 | 0.179 | |||
| Histological grade I-II vs. III-IV |
3.389 | 2.169-5.881 | < 0.001 | 2.817 | 1.741-4.441 | 0.004 |
| Lymph node Presence vs. Absence |
5.417 | 3.012-8.118 | 0.012 | 3.341 | 2.367-4.615 | 0.026 |
| Distant metastasis Presence vs. Absence |
5.361 | 3.016-9.558 | 0.002 | 3.561 | 2.871-7.554 | 0.018 |
| miR-495 low vs. high |
3.515 | 2.376-7.663 | 0.006 | 3.147 | 2.218-6.225 | 0.011 |
Table 2. Prognostic factors in Cox proportional hazards model.
Correlation between miR-495 expression and overall survival
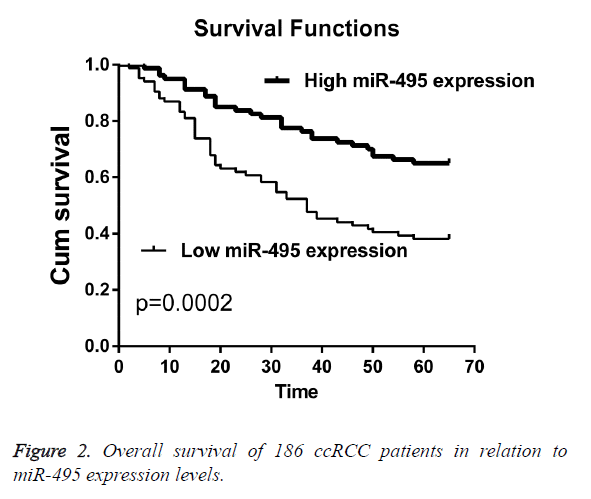
The prognostic value of miR-495 expression for overall survival in ccRCC patients was evaluated by comparing the patients with high and low miR-495 expression. Kaplan-Meier survival analysis and log-rank test demonstrated that patients with low expression of miR-495 had significantly worse overall survival rates compared with those who had cancers with low miR-495 expression (p=0.0002). Univariate and multivariate analyses were utilized to evaluate whether the miR-495 expression level was independent prognostic parameters of patient outcomes. The results showed that the expression of miR-495 was an independent prognostic factor for overall patient survival (p=0.011, Table 2).
Discussion
Renal cell carcinoma remains to be one of the leading causes of death [13]. It is necessary to search for novel markers for ccRCC, which could improve the outcome of this lethal disease. MiRNAs are aberrant expressions in multiple tumors and play an important role in tumorigenesis and development and may be used as novel biomarkers for the prognosis and treatment of cancer. For instance, Bai et al. found that down-regulation of miR-32 predicted poor prognosis in human non-small cell lung cancer [14]. Zhang et al. showed that MicroRNA-377 suppressed proliferation and invasion of human glioblastoma cells by directly targeting specificity protein 1 [15]. Zhang et al. identified Serum miR-200c as a prognostic biomarker for gastric cancer [16]. Zhu et al. found that MiR-451 acts as an anti-oncogene in RCC and the down-regulation of miR-451 was correlated with lower survival rate of RCC patients [17]. In our present study, our attention focuses on miR-495.
In our present study, we showed that miR-495 was significantly down-regulated in ccRCC tissues for the first time. The relationship of the miR-495 with various clinical features of ccRCC was analyzed. The results showed that low expression of miR-495 was correlated with higher histological grade, lymph node metastasis and tumor distant metastasis. Suggesting that miR-495 might be involved in the carcinogenesis and metastasis of ccRCC. Furthermore, ccRCC patients with low miR-495 expression level had distinctly shorter OS than patients with high miR-495 expression level. The results of Cox regression analyses revealed that miR-495 may be an independent prognostic marker for ccRCC patients.
Several studies reported that miR-495 acted as a tumor suppressor gene or an oncogene in a lot of cancers. For instance, miR-495 functions as a tumor suppressor in acute myeloid leukemia (AML) by targeting essential leukemia-related genes [18]. Xu et al. found that MicroRNA-495 suppressed cell growth and migration in endometrial cancer by targeting FOXC1 [19]. Another study revealed that miR-495 acts as an oncogene in breast cancer via down regulation of E-cadherin and REDD1 [20]. To our interest, Lv et al. found that MicroRNA-495 served as a tumor suppressor in human ccRCC, suggesting that miRNA-495 could be promising biomarkers for ccRCC prognosis.
Conclusion
We provide the evidence that miR-495 was down regulated in ccRCC patients and may act as independent prognostic factors for ccRCC patients.
References
- White NM, Yousef GM. MicroRNAs: exploring a new dimension in the pathogenesis of kidney cancer. BMC Med 2010; 8: 1-4.
- van Spronsen DJ. Novel treatment strategies in clear-cell metastatic renal cell carcinoma. Anti-Cancer Drugs 2005; 16(7): 709-717.
- Lipworth L, RE Tarone, Mclaughlin JK. The epidemiology of renal cell carcinoma. Eur Urol 2006; 60: 2353-2358.
- Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet 2009; 373: 1119-1132.
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281-297.
- Ambros V. The functions of animal microRNAs. Nature 2004; 431: 350-355.
- Zhang B. microRNAs as oncogenes and tumor suppressors. Developmental Biol 2005; 353: 1768-1771.
- Fabbri M, Croce CM, Calin GA. MicroRNAs. Cancer J 2008; 14: 1-6.
- Li Z, Zhang G, Li D, Jie Z, Chen H, Xiong J, Liu Y, Cao Y, Jiang M, Le Z, Tan S. Methylation-associated silencing of miR-495 inhibit the migration and invasion of human gastric cancer cells by directly targeting PRL-3. Biochem Biophysical Res Commun 2015; 456: 344.
- Chu H, Chen X, Wang H, Du Y, Wang Y, Zang W, Li P, Li J, Chang J, Zhao G, Zhang G. MiR-495 regulates proliferation and migration in NSCLC by targeting MTA3. Tumour Biol 2014; 35: 3487-3494.
- Cao M, Nie W, Li J, Zhang Y, Yan X, Guan X, Chen X, Zen K, Zhang CY, Jiang X, Hou D. MicroRNA-495 induces breast cancer cell migration by targeting JAM-A. Protein Cell 2014; 5: 862-872.
- Lv C, Bai Z, Liu Z, Luo P, Zhang J. MicroRNA-495 suppresses human renal cell carcinoma malignancy by targeting SATB1. Am J Transl Res 2015; 7: 1992-1999.
- Huang Y, Dai Y, Yang J, Chen T, Yin Y, Tang M, Hu C, Zhang L. Microarray analysis of microRNA expression in renal clear cell carcinoma. Eur J Surg Oncol 2009; 35: 1119-1123.
- Bai Y. Expression of miR-32 in human non-small cell lung cancer and its correlation with tumor progression and patient survival. Int J Clin Exp Pathol 2014; 8: 824-829.
- Zhang R. MicroRNA-377 inhibited proliferation and invasion of human glioblastoma cells by directly targeting specificity protein 1. Neurooncol Pract 2014; 16: 1510.
- Zhang HP, Sun FB, Li SJ. Serum miR-200c expression level as a prognostic biomarker for gastric cancer. Genetics Mol Res 2015; 14: 15913.
- Zhu S, Huang Y, Su X. Mir-451 correlates with prognosis of renal cell carcinoma patients and inhibits cellular proliferation of renal cell carcinoma. Med Sci Monit 2016; 22: 183.
- Jiang X. MiR-495 is a tumor-suppressor microRNA down-regulated in MLL-rearranged leukemia. Proceedings of the National Academy of Sciences of the United States of America 2012; 109: 19397.
- Xu YY. MicroRNA-495 downregulates FOXC1 expression to suppress cell growth and migration in endometrial cancer. Tumor Biol 2016; 37: 239.
- Hwangverslues WW. MiR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via downregulation of E-cadherin and REDD1. Oncogene 2011; 30: 2463.