Research Article - Biomedical Research (2017) Volume 28, Issue 1
Decimeter wave mechanisms in promoting peripheral nerve regeneration
Jiang-Bo Bai1, Ying-Ze Zhang2, Kun-Lun Yu1, Hong-Fang Zhao1 and De-Hu Tian1,2*1Department of Hand Surgery, the Third Hospital of Hebei Medical University, PR China
2Hebei Provincial Key Laboratory of Orthopedic Biomechanics, Shijiazhuang 050051, Hebei Province, PR China
- *Corresponding Author:
- De-Hu Tian
Department of Hand Surgery
The Third Hospital of Hebei Medical University, PR China
Accepted date: June 02, 2016
Abstract
Objective: The aim of our study was to investigate the effect of decimeter waves on nerve healing in a rat peripheral nerve injury model.
Method: Twenty-four hours after the operation, the experimental group (40 rats) was treated with decimeter waves. Another 40 rats that did not undergo decimeter wave therapy were assigned as the control group. Gross and light microscopy assessments were performed on the 7th, 14th, 30th, 60th and 90th day after the operation. Electron microscopy analysis was performed on the 14th, 30th and 60th day. Axon image analysis and electrophysiological assessment of sciatic nerves were performed on the 90th day after the operation. Sciatic nerve function was evaluated using the walking track analysis on the 30th, 60th and 90th day after the operation.
Result: Histological analysis revealed that decimeter waves can increase local blood circulation, inhibit inflammation infiltration, and reduce perineural adhesions. Moreover, decimeter waves can promote axon regeneration and the remyelination of injured nerves, as well as the maturation of regenerated nerve structures. Electron microscopy, axon image, electrophysiological and walking track analyses revealed that the decimeter wave group had more myelinated nerve fiber counts, larger mean axon diameter and myelin sheath thickness, shorter latency of compound muscle action potential, faster nerve conduction velocity and higher wave amplitude, and better sciatic functional index recovery rates, when compared to the control group (all P<0.01).
Conclusion: Our findings suggest that decimeter waves can promote the regeneration of peripheral nerves and functional recovery after peripheral nerve injury. However, further studies are warranted.
Keywords
Decimeter wave, Peripheral nerve injury, Nerve regeneration, Sciatic functional index.
Introduction
Peripheral nerve injuries are common clinical problems that can result from mechanical factors such as crushing, cutting or stresses, as well as ischemic, radial, or chemical factors [1]. Following peripheral nerve injuries, huge histopathological changes may occur in both the distal and proximal sections of the nerve. With the advancement and spread of microsurgical techniques, the success rate of nerve recovery in peripheral nerve injuries has greatly increased. The superb technologies of microsurgical repair have provided a good foundation for nerve regeneration. However, the recovery of neural function remains unsatisfactory in most cases. In fact, the complete functional repair of nerve function is almost not possible; and partial recovery of nerve function remains unsatisfactory in most cases [2].
In recent years, various efforts have been devoted in maximizing the recovery of the function of injured peripheral nerves. Biological stimulation such as bioactive molecules and related drugs has been used to improve peripheral nerve repair [3-5]. On this basis, physiotherapy has currently become a research focus in the field of peripheral nerve repair and regeneration. Previous studies have shown that physical stimulation such as electric stimulation, ultrasound, laser stimulation, millimeter wave and modulated mediumfrequency pulsed electromagnetic stimulation could promote nerve regeneration and repair after peripheral nerve injury [6-15]. In early clinical studies, we found that the decimeter wave had a good curative effect on nerve repair in patients with diabetic peripheral neuropathy.
However, to date, few studies have been reported on the effect of decimeter waves on peripheral nerve regeneration. Therefore, a chamber model of rat sciatic nerve regeneration was constructed in this study; and the partially injured nerve was radiated by decimeter waves. We investigated the effect of the decimeter wave on peripheral nerve repair and regeneration following peripheral nerve injury through gross observation, light microscopy, transmission electron microscopy, axon image analysis, and electrophysiological and Sciatic Function Index (SFI) analysis.
Materials and Methods
All experimental procedures were approved by the Animal Study Ethics Committee of the Third Hospital of Hebei Medical University and were performed in accordance with the institutional criteria for the care and use of laboratory animals in research.
Animals and experimental groups
A total of 80 Sprague-Dawley (SD) rats (male or female) weighing 200-250 g were used in this experimental study. These rats were randomly divided into two groups (each group, n=40): decimeter wave group, and control group. Animals were fasted with water for eight hours before the operation, and were anesthetized with 1% sodium pentobarbital (30 mg/kg) by intraperitoneal injection. A posterior median incision was made on the right thigh. After separating the intermuscular space between the biceps femoris, semitendinosus and semimembranosus muscles, the sciatic nerve was exposed. The sciatic nerve was resected 10 mm distal to the piriformis inferior margin with retraction at the two broken ends. Under the guidance of an 8× surgical microscope, two nerve stumps were inserted into the silicon tube (length, 10 mm; inner diameter, 1.5 mm) using 9-0 sutures to form a nerve regeneration chamber. A 6 mm gap was formed between the two stumps within the silicon tube. After thorough hemostasis, the intermuscular space was closed; and the skin incision was sutured. All procedures of this experiment were performed by the same physician and assistant. After the operation, rats were placed under warm light to recover from anesthesia, and housed in separate cages with free access to food and water, room temperature at 21-27°C, 40-50% humidity, and a 07:19 light-dark cycle.
Perioperative management
One day after the operation, rats were fixed in the prostrate position on a table, and their right posterior thighs were exposed to decimeter waves every day until the 90th days after the operation. The TMA-A double-frequency microwave hyperthermia apparatus (Beijing Electronic Medical Instrument Factory, Beijing, China) was applied to the incision site of the rat through a probe. Parameters were as follows: frequency, 915 MHz; power, 5 W; radiating distance, 10 cm. Microwave treatment was conducted once a day for five consecutive days per week, until the rats were sacrificed. Treatment duration was 10 minutes per session. Rats in the control group were also fixed on a table in the prostrate position for 10 minutes, but these rats did not receive decimeter wave therapy.
Gross observation and histological evaluation
Dissection was carried out on the 7th, 14th, 30th, 60th and 90th day post-operation. The bridge connection segment of the sciatic nerve was exposed. Then, gross observation of the lesions of the sciatic nerves and its surrounding tissues and nerve adhesion in rats were conducted. Neural segments (2 mm) were obtained from the proximal and distal end of the bridge connection segment, followed by 10% formalin fixation and hematoxylin and eosin (H&E) staining. The nerve structure was observed under light microscopy (CX31; Olympus, Japan).
Electron microscopic evaluation
At the 13th, 30th and 60th day after the operation, samples of the sciatic nerve were obtained and fixed with a 3% Glutaraldehyde (GA) solution; and embedded with resin. Thereafter, ultra-thin sections were obtained to observe neural regeneration conditions under transmission electron microscopy (TEM, H-7500; HITACHI, Japan).
Image analysis of the axon
On the 90th day post-operation, specimens from the osmium tetroxide-stained distal end of the nerve stumps were analyzed using an image analyzer (IBAS 2000; Zeiss Kontron, Munich, Germany). The number of myelinated nerve fibers, axon diameter, and myelin sheath thickness were calculated.
Electrophysiological evaluation
On the 90th day after the operation, Compound Muscle Action Potential (CMAP) was detected and evaluated using a myoelectricity evoked potential apparatus (DISA1500, Denmark). The following parameters were measured and recorded: latency, amplitude, and nerve conduction velocity.
Sciatic function index evaluation
The evaluation of sciatic nerve function in animals was performed using the walking track analysis at the 30th, 60th and 90th day after the operation. A walk box (length: 50 cm, and width: 8.5 cm) was made with one port opened at one side. A white sheet of paper was spread at the bottom of the walk box. The plantar hind feet of rats were pigmented with carbon black ink. Thereafter, the rats were placed at one side of the walk box, and were induced to walk to the other side of the walk box. The sciatic functional index (SFI) and recovery rates were obtained according to a previous report [16]. SFI = 0 for normal, and -100 for complete injury.
Statistical analysis
Statistical analyses were performed using the statistical software SSPS 11.0 (SPSS Inc., Chicago, USA). Data were expressed as arithmetic means ± standard deviation. All count data were compared with T-test analysis and p<0.05 was considered as statistical significance in the statistical analysis.
Results
Gross and histological evaluation
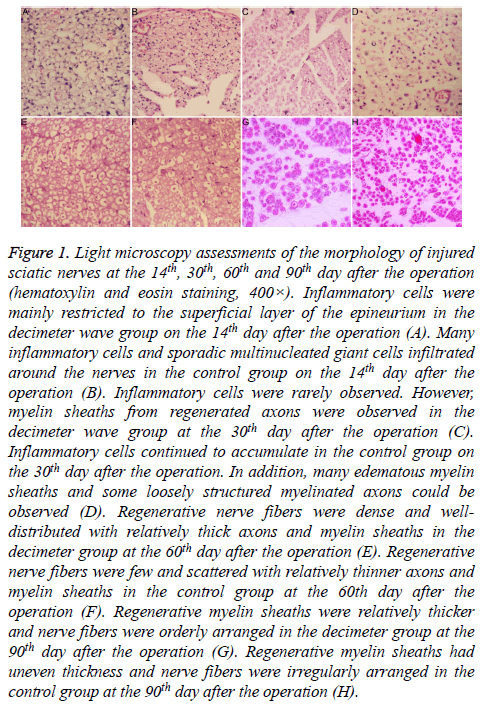
On the 7th and 14th day after the operation, hyperemia and edema were noted in subcutaneous tissues and tissues surrounding the nerves in both groups. These changes were relatively smaller in the decimeter wave group, compared to the control group. Perineural adhesions in the decimeter wave group were limited and looser compared to the control group. Perineural adhesions in the control group were easily separated bluntly. In the decimeter wave group, inflammatory cells were restricted to the superficial layer of the epineurium; while many inflammatory cells and interspersed multinucleated giant cells had infiltrated tissues around the nerve stumps in the control group (Figures 1A and 1B). Furthermore, regenerated axons were observed in the middle portion of the regenerated nerves in the decimeter group, but these did not span across the distal anastomosis.
Figure 1: Light microscopy assessments of the morphology of injured sciatic nerves at the 14th, 30th, 60th and 90th day after the operation (hematoxylin and eosin staining, 400×). Inflammatory cells were mainly restricted to the superficial layer of the epineurium in the decimeter wave group on the 14th day after the operation (A). Many inflammatory cells and sporadic multinucleated giant cells infiltrated around the nerves in the control group on the 14th day after the operation (B). Inflammatory cells were rarely observed. However, myelin sheaths from regenerated axons were observed in the decimeter wave group at the 30th day after the operation (C). Inflammatory cells continued to accumulate in the control group on the 30th day after the operation. In addition, many edematous myelin sheaths and some loosely structured myelinated axons could be observed (D). Regenerative nerve fibers were dense and welldistributed with relatively thick axons and myelin sheaths in the decimeter group at the 60th day after the operation (E). Regenerative nerve fibers were few and scattered with relatively thinner axons and myelin sheaths in the control group at the 60th day after the operation (F). Regenerative myelin sheaths were relatively thicker and nerve fibers were orderly arranged in the decimeter group at the 90th day after the operation (G). Regenerative myelin sheaths had uneven thickness and nerve fibers were irregularly arranged in the control group at the 90th day after the operation (H).
On the 30th and 60th day after the operation, hyperemia and edema of the tissues surrounding the nerves almost abated in both groups (Figures 1C-1F). In the decimeter wave group, loose perineural adhesions were present; while in the control group, extensive dense adhesions were present, which were difficult to separate bluntly. In the decimeter wave group, inflammatory cells were rarely observed; while in the control group, a small number of inflammatory cells continued to accumulate. Regenerated axons all spanned across the distal anastomosis and entered into the distal nerve in both groups. In the decimeter wave group, myelin sheaths in the regenerated axons could be observed; and was thicker than that in the control group.
On the 90th day after the operation, surfaces of the nerves in the decimeter wave group appeared smooth; and revealed only few filamentous adhesions or even no adhesion to their surrounding tissues. In the control group, perineural adhesions were obviously loose compared to that at the 30th and 60th day after the operation. Regenerated nerves in both groups were obviously thicker than that at the 60th day postoperatively, which was close to the diameter of the proximal nerve trunk. Regenerated neural fibers in the decimeter wave group were dense and orderly arranged with rough diameters; while these were rare in the control group and arranged disorderly with uneven diameters (Figures 1G and 1H).
Electron microscopic evaluation
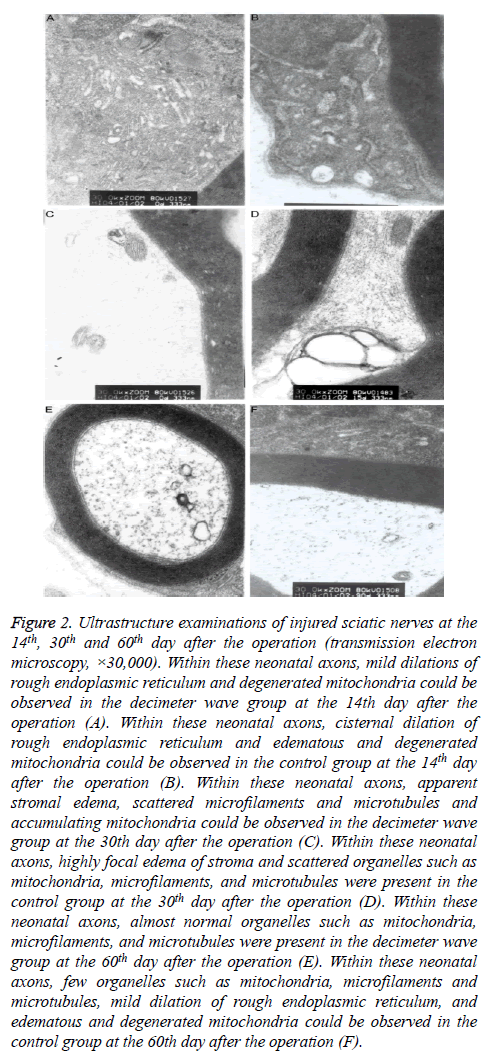
On the 14th day after the operation, many neonatal axon buds encapsulated by Schwann cells were present at the proximal end of the nerve fibers in the decimeter wave group. Within the neonatal axon bud, few organelles such as mitochondria, microfilaments, microtubules and vesicles were present. In the control group, the myelin sheath was distributed unevenly, and the structure was in disorder (Figures 2A and 2B). On the 30th day after the operation, the myelin sheaths were relatively thicker and the regenerated axons appeared normal in the decimeter wave group. Some almost normal organelles such as mitochondria, microfilaments, and microtubules could be observed. In the control group, a disordered structure remained. There were few irregularly-arranged thin myelin sheaths (Figures 2C and 2D). On the 60th day after the operation, the decimeter wave group had a large quantity of regenerated myelinated fibers, which were orderly arranged. The fascicular structure was evident. The myelinated fibers had relatively larger axon diameters. Furthermore, the myelin sheath was thick with a clear structure (Figure 2E), while the myelin sheath had an uneven thickness with an immature structure in the control group (Figure 2F).
Figure 2: Ultrastructure examinations of injured sciatic nerves at the 14th, 30th and 60th day after the operation (transmission electron microscopy, ×30,000). Within these neonatal axons, mild dilations of rough endoplasmic reticulum and degenerated mitochondria could be observed in the decimeter wave group at the 14th day after the operation (A). Within these neonatal axons, cisternal dilation of rough endoplasmic reticulum and edematous and degenerated mitochondria could be observed in the control group at the 14th day after the operation (B). Within these neonatal axons, apparent stromal edema, scattered microfilaments and microtubules and accumulating mitochondria could be observed in the decimeter wave group at the 30th day after the operation (C). Within these neonatal axons, highly focal edema of stroma and scattered organelles such as mitochondria, microfilaments, and microtubules were present in the control group at the 30th day after the operation (D). Within these neonatal axons, almost normal organelles such as mitochondria, microfilaments, and microtubules were present in the decimeter wave group at the 60th day after the operation (E). Within these neonatal axons, few organelles such as mitochondria, microfilaments and microtubules, mild dilation of rough endoplasmic reticulum, and edematous and degenerated mitochondria could be observed in the control group at the 60th day after the operation (F).
Axon image analysis
On the 90th day after the operation, distal segments of the regenerative nerve in the decimeter wave group had more myelinated nerve fibers counts, a larger mean axon diameter and myelin sheath thickness compared to the control group (all P<0.01) (Table 1).
| Group | Number of myelinated nerve fibers (n/mm2) | Axon diameter (μm) | Myelin sheath thickness (μm) |
|---|---|---|---|
| Decimeter wave group | 3,624 ± 417.83 | 3.96 ± 0.24 | 1.46 ± 0.12 |
| Control group | 2,018 ± 403.26 | 2.78 ± 0.33 | 0.86 ± 0.16 |
| P | <0.01 | <0.01 | <0.01 |
Table 1. The number of myelinated nerve fibers, axon diameter and myelin sheath thickness in the decimeter wave group and control group in the axon image.
Electrophysiological evaluation
On the 90th day after the operation, electrophysiological analysis showed that the decimeter wave group had a shorter latency of compound muscle action potential, faster nerve conduction velocity and higher wave amplitude, when compared to the control group (all P<0.01) (Table 2).
| Group | Latency (ms) | Nerve conduction velocity (m/s) | Wave amplitude (mV) |
|---|---|---|---|
| Decimeter wave group | 2.14 ± 0.86 | 46.29 ± 5.14 | 6.28 ± 2.62 |
| Control group | 3.21 ± 1.04 | 20.68 ± 6.32 | 3.65 ± 1.76 |
| P | <0.01 | <0.01 | <0.01 |
Table 2. Electrophysiological Data in the decimeter wave group and control group.
Function evaluation of the sciatic nerve
The decimeter wave group had significantly better SFI recovery rates on the 30th, 60th, 90th day after the operation compared to the control group (all P<0.01) (Table 3).
| Groups | 30th day postoperatively | 60th postoperatively | 90th postoperatively |
|---|---|---|---|
| Decimeter wave group | 23.42 ± 3.12 | 36.21 ± 6.24 | 43.26 ± 4.12 |
| Control group | 15.16 ± 3.24 | 24.24 ± 5.78 | 30.16 ± 3.49 |
| P | <0.01 | <0.01 | <0.01 |
Table 3. Sciatic function index (SFI) of the decimeter wave group and control group after the operation.
Discussion
After peripheral nerve injury, local hyperemia, edema and extensive and dense adhesions with peripheral tissues can result in the further formation of entrapment; and thereby aggravate hyperemia, induce edema, promote regenerated nerve ischemia and hypoxia, and finally hinder nerve regeneration. According to previous reports, decimeter wave therapy could inhibit inflammatory reactions after injuries, improve local circulation in the injured site, enhance the metabolism of the injured area, and reduce scar formation; and thereby alleviate adhesion [17-19]. In this study, our findings revealed that hyperemia and edema were noted in subcutaneous tissues and tissues surrounding the nerves in both groups at the 7th day after surgery; which was relatively smaller in the decimeter wave group, compared to the control group.
Furthermore, we observed that inflammatory cells were restricted to the superficial layer of the epineurium in the decimeter wave group, while many inflammatory cells and interspersed multinucleated giant cells had infiltrated tissues around the nerve stumps in the control group at the 14th day after the operation. At the 30th day after operation, loose perineural adhesions were present in the decimeter wave group; while extensively dense adhesions that were difficult to separate bluntly were present in the control group. In addition, inflammatory cells were rarely observed in the decimeter wave group; while a small number of inflammatory cells continued to accumulate in the control group. These findings suggest that the decimeter wave could effectively inhibit inflammation after injury, improve local blood circulation, reduce scar formation, and alleviate nerve adhesion; and thereby contributing to nerve regeneration.
The process of nerve repair and regeneration following peripheral nerve injury was aimed at allowing axons of the injured proximal nerve to grow into the distal nerve. The successful repair and regeneration of peripheral nerve injury include the degree of sprouting and the elongation of proximal axons within the nerves [20,21]. Therefore, the number of axons and degree of maturity of regenerated nerves can reflect the regeneration condition of the injured nerves [22]. In the present study, electron microscopy evaluation revealed that many neonatal axon buds existed at the proximal end of the nerve fibers at the 14th day after surgery in decimeter wave group. Within these neonatal axon buds, few organelles such as mitochondria, microfilaments, microtubules and vesicles were present. In contrast, we did not observe neonatal axon buds in the control group. At the 30th day after surgery, myelin sheaths in the decimeter wave group were relatively thicker, and the regenerated axons appeared normal. Some almost normal organelles such as mitochondria, microfilaments, and microtubules could be observed. In the control group, a disordered structure remained. Furthermore, there were few irregularly-arranged thin myelin sheaths. The axon image analysis revealed that myelinated nerve fiber counts, the myelinated nerve fiber diameter and myelin sheath thickness in the decimeter wave group were all significantly greater than in the control group. Thus, the degree of regenerated nerve maturation in the decimeter wave group was significantly higher than in the control group. Regardless of this, the conclusion that the decimeter wave group is superior to the control group with regard to the quality of nerve regeneration remains inadequate. This is due to many small neonatal buds derived from proximal nerves that may stop at the scar tissue, neuroma, or even the sensory nerve ending after growing over damaged nerves. These regenerated axons fail to reach their specific target organs, and does not help to the recovery of neural function. Furthermore, these only affect the number of the nerve fibers and the diameter of the cross section; and further affect the actual value of the morphological evaluation. On this occasion, the assessment of the quality of nerve regeneration would be more perfect, accurate and reliable if electrophysiological and SFI assessments were used in combination.
Peripheral nerve regeneration is highly associated with the repair of neural function, especially nerve conduction velocity, conduction latency and potential amplitude [23]. In this study, electrophysiological analysis revealed that both groups had different degrees of functional recovery for regenerated nerves. Furthermore, the decimeter wave group had shorter latency of compound muscle action potential, faster nerve conduction velocity and higher wave amplitude, when compared to those in the control group; which demonstrate that neuromuscular function in the decimeter wave group were better than that in the control group. SFI is a direct behavioral indicator for the recovery of sciatic neural function, and has long been the standard method for assessing motor recovery in the models of rat sciatic nerves [4,24]. In this study, we found that the decimeter wave group had significantly better SFI recovery rates after the operation, compared to the control group. All of above findings suggest that the decimeter wave played an important role in promoting the functional recovery of regenerated nerves. The underlying mechanisms can be explained from the tissue structure of the regenerated nerve; that is, the decimeter wave can improve local blood circulation, inhibit inflammatory response, and relieve perineural adhesion and entrapment. This would provide a good microenvironment for nerve regeneration, which would help in axonal regeneration and remyelination, and promote the functional recovery.
This study has several limitations. The observation that decimeter wave therapy promotes nerve regeneration in this study was based on the perspective of histopathology. Further experimental studies are needed to identify the molecular basis that underlies the effects of decimeter wave therapy on peripheral nerve regeneration. For potential clinical applications, additional research are necessary to confirm whether the decimeter wave therapy has no adverse effects and yields the same results in humans as it did in our animal model.
References
- Yuksel TN, Halici Z, Demir R, Cakir M, Calikoglu C, Ozdemir G. Investigation of the effect of telmisartan on experimentally induced peripheral nerve injury in rats. Int J Neurosci 2015; 125: 464-473.
- Anders JJ, Geuna S, Rochkind S. Phototherapy promotes regeneration and functional recovery of injured peripheral nerve. Neurol Res 2004; 26: 233-239.
- Mohammadi R, Mehrtash M, Nikonam N, Mehrtash M, Amini K. Ketoprofen combined with artery graft entubulization improves functional recovery of transected peripheral nerves. J Craniomaxillofac Surg 2014; 42: 2076-2081.
- Kaplan T, Kafa IM, Cansev M, Bekar A, Karli N, Taskapilioglu MO. Investigation of the Dose-Dependency of Citicoline Effects on Nerve Regeneration and Functional Recovery in a Rat Model of Sciatic Nerve Injury. Turk Neurosurg 2014; 24: 54-62.
- Farahpour MR, Ghayour SJ. Effect of in situ delivery of acetyl-L-carnitine on peripheral nerve regeneration and functional recovery in transected sciatic nerve in rat. Int J Surg 2014; 12: 1409-1415.
- Lv Y, Zhao P, Chen G, Sha Y, Yang L. Effects of low-intensity pulsed ultrasound on cell viability, proliferation and neural differentiation of induced pluripotent stem cells-derived neural crest stem cells. Biotechnol Lett 2013; 35: 2201-2212.
- Kim JR, Oh SH, Kwon GB, Namgung U, Song KS, Jeon BH. Acceleration of peripheral nerve regeneration through asymmetrically porous nerve guide conduit applied with biological/physical stimulation. Tissue Eng Part A 2013; 19: 2674-2685.
- Jahromy FZ, Behnam H, Mansoori K, Rahimi AA, Edalat R, Mobarake JI. The effect of ultrasound on the expression of CNTF gene, a possible cause of ultrasound influence on the rate of injured peripheral nerve regeneration. Australas Phys Eng Sci Med 2013; 36: 323-329.
- Hoang NS, Sar C, Valmier J, Sieso V, Scamps F. Electro-acupuncture on functional peripheral nerve regeneration in mice: a behavioural study. BMC Complement Altern Med 2012; 12: 141.
- Anders JJ, Moges H, Wu X, Erbele ID, Alberico SL, Saidu EK. In vitro and in vivo optimization of infrared laser treatment for injured peripheral nerves. Lasers Surg Med 2014; 46: 34-45.
- Beck-Broichsitter BE, Lamia A, Geuna S, Fregnan F, Smeets R, Becker ST. Does pulsed magnetic field therapy influence nerve regeneration in the median nerve model of the rat? Biomed Res Int 2014; 2014: 401760.
- Shen CC, Yang YC, Huang TB, Chan SC, Liu BS. Neural regeneration in a novel nerve conduit across a large gap of the transected sciatic nerve in rats with low-level laser phototherapy. J Biomed Mater Res A 2013; 101: 2763-2777.
- Mendonca AC, Barbieri CH, Mazzer N. Directly applied low intensity direct electric current enhances peripheral nerve regeneration in rats. J Neurosci Methods 2003; 129: 183-190.
- Kelleher MO, Al-Abri RK, Lenihan DV, Glasby MA. Use of a static magnetic field to promote recovery after peripheral nerve injury. J Neurosurg 2006; 105: 610-615.
- Park SC, Oh SH, Seo TB, Namgung U, Kim JM, Lee JH. Ultrasound-stimulated peripheral nerve regeneration within asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. J Biomed Mater Res B Appl Biomater 2010; 94: 359-366.
- Bain JR, Mackinnon SE, Hunter DA. Functional-Evaluation of Complete Sciatic, Peroneal, and Posterior Tibial Nerve Lesions in the Rat. Plast Reconstr Surg 1989; 83: 129-136.
- Tian D, Zhao M, Wang L. Effect of enhancing peripheral nerve regeneration with combined physical factors. Chin J Rehabil Med 2007.
- Zhang Y, Tian D, Zhang Y. Observation of the therapeutic effects of comprehensive rehabilitation on peripheral nerve complete impairment. Chin J Rehabil Med 2008; 11: 15.
- Gupta R, Rowshan K, Chao T, Mozaffar T, Steward O. Chronic nerve compression induces local demyelination and remyelination in a rat model of carpal tunnel syndrome. Exp Neurol 2004; 187: 500-508.
- English AW, Cucoranu D, Mulligan A, Rodriguez JA, Sabatier MJ. Neurotrophin-4/5 is implicated in the enhancement of axon regeneration produced by treadmill training following peripheral nerve injury. Eur J Neurosci 2011; 33: 2265-2271.
- Hausner T, Pajer K, Halat G, Hopf R, Schmidhammer R, Redl H. Improved rate of peripheral nerve regeneration induced by extracorporeal shock wave treatment in the rat. Exp Neurol 2012; 236: 363-370.
- Xu XY, Yee WC, Hwang PYK, Yu H, Wan ACA, Gao SJ. Peripheral nerve regeneration with sustained release of poly(phosphoester) microencapsulated nerve growth factor within nerve guide conduits. Biomaterials 2003; 24: 2405-2412.
- Wang YD, Liu YG, Liu Q. Ganglioside promotes the bridging of sciatic nerve defects in cryopreserved peripheral nerve allografts. Neural Regen Res 2014; 9: 1820-1823.
- Tamaddonfard E, Farshid AA, Maroufi S, Kazemi-Shojaei S, Erfanparast A, Asri-Rezaei S. Effects of safranal, a constituent of saffron, and vitamin E on nerve functions and histopathology following crush injury of sciatic nerve in rats. Phytomedicine 2014; 21: 717-723.