Research Article - Biomedical Research (2017) Volume 28, Issue 10
Daptomycin recovers stroke injury and advances blood-brain barrier in rat brain
Chunshui Yang1,2*, Jianqing Pan1,2, Chuanming Wang1,2, Wenfei Li1,2 and Lijie Ren3
1Department of Neurology, the Affiliated Shenzhen Nanshan Hospital, Shenzhen University, Shenzhen, Guangdong Province, PR China
2Stroke Center, the Affiliated Shenzhen Nanshan Hospital, Guangdong Medical College, Shenzhen, Guangdong Province, PR China
3Department of Neurology, Shenzhen Second People's Hospital, Shenzhen, Guangdong Province, PR China
- *Corresponding Author:
- Chunshui Yang
Stroke Center
The Affiliated Shenzhen Nanshan Hospital
Guangdong Medical College, PR China
Accepted on February 28, 2017
Abstract
Background: Daptomycin (DPM) an antibiotic of lipopeptide class has been used in the treatment of infections caused by gram-positive bacteria. DPM interferes with Matrix Metalloproteinases (MMPs) activity the enzyme responsible for brain damage. However effects of DPM in early treatments of brain injury followed by repurfusion are not well studied. The aim of current research was to evaluate the consequences of DPM administration in early stage of stroke followed by perfusion in rats induced to stroke, and effects on Blood Brain Barrier (BBB) and neuro-inflammation were studied. The study was guided by MRI studies, evaluation of histology and biochemical parameters.
Methods: For present study 30 adult rats spontaneously hypertensive were subjected to 90 minutes of transient Middle Cerebral Artery Occlusion (MCAO). In very early stage the rats received repurfusion and also one group of rats were administered a unit dose of DPM (50 mg/kg) and the other group only received vehicle. The rats were studied at defined time points for upto 4 weeks with MRI.
Results: MRI studies showed DPM reduced infract size and also prevented brain tissue damages compared to vehicle treated rats in ischemic zones after two and four weeks of stroke. Arterial Spin Labeling (ASL) showed DPM improved Cerebral Blood Flow (CBF) and perfusion. Results of dynamic contrast-enhanced MRI demonstrated that DPM decreased permeability of BBB which was associated with elevated levels of TJPs when assessed with Western blot. Mediators of inflammation were examined by Immunohistochemistry. The findings suggested DPM reduced the inflammatory reaction by modulating inflammatory mediators (IL 1β, IL 6, IL 10 and TNF α) and NF κB (p65) due to ischemic injury.
Conclusions: DPM treatment at very early stage of stroke followed by reperfusion significantly encouraged neurovascular remodeling and attenuated stroke injury by decreasing brain tissue loss, advancing blood brain barrier and reducing overall inflammatory response.
Keywords
Daptomycin, Stroke, Blood brain barrier, Neuro-inflammation.
Introduction
Stroke has been documented to be the third highest cause for death in many industrialized nations [1] it has been also been cause for permanent disability globally [2]. Acute ischemic stroke has been the most common form of stroke which results due to emboli causing sudden occlusion of blood vessels which leads to immediate deprivation of valuable oxygen and glucose to the brain. Findings suggest number of mechanisms responsible for causing stroke with ischemic injury and inflammation being the major in its progression [3]. Cerebral ischemia triggers series of pathological events such as vasogenic cerebral oedema followed by disruption of Blood Brain Barrier (BBB), intracranial hemorrhage leading to neuronal death, as a consequence within minutes of cerebral ischemia an irreversible damage to brain occurs [4].
Reports suggest different pathophysiology’s of stroke which are accompanied with number of complex factors, which include excitotoxicity, oxidative stress, inflammation or apoptosis [5]. Matrix Metalloproteinases (MMPs) have been suggested as one of the main factors responsible for cell death and disrupting BBB in early stage of stroke. Reports suggest inhibition of MMP can lead to designing new therapies in treating brain injuries due to cerebral ischemia [6-8].
Literatures indicate supportive role of MMPs inhibitors in rat models for middle cerebral artery occlusion [6,9-10].
With inflammation being one of the mechanisms [11-13] it is characterized by production of inflammatory cytokines subsequent to ischemic injury. Inflammation is reported to worsen damage of brain tissues by microglial activation, macrophages of resident perivascular and parenchymal and also inflammatory cells infiltration. Factors related to inflammation mediated stroke such as Tumor Necrosis Factor (TNF)-α, Cytokines such as IL-6, IL-10, and (IL)-1β, transforming growth factor-β (TGF-β) [14-16]. The factor like TNF-α has been found to be a very crucial factor in regulation of body’s inflammatory and immune response; inhibition of it can lead to improvement of stroke [17].
Interleukin-1 (IL-1) a member of cytokine family has been found to be involved in host defense. It has been found to be associated in protein synthesis, fever, inflammation and immune response. IL-1 is elevated after permanent cerebral ischemia leading to activation and development of astrocytes and glial cells [18]. IL-1 induces astrocytes for secretion of IL-6, the pleiotropic IL-6 is expressed majorly after stroke [19]; however it is still unclear about the inflammatory status of IL-6. Interleukin (IL-10) the other member is found to be a cytokine which inhibits the expression of TNF-α and IL-1. The activation of pleiotropic factor NF-κB leads to transcription of some specific genes triggering release of inflammation causing cytokines.
Daptomycin is an antibiotic belonging to lipopeptide class it is used in the treatment of infections caused by Gram-positive organisms. The neuroprotective activity of Daptomycin has been contributed to its ability to interfere with MMP activity, studies confirmed that Daptomycin decreased the levels of MMPs the enzyme responsible for brain damage [20]. Antibiotics have been found to affect the balance of pro- and anti-inflammatory cytokines [21]. In an experiment involving various antibiotics on peripheral mononuclear cells Pichereau et al. established that daptomycin caused decreased production of cytokines in vitro after exposure to toxins [22]. In a study done by English et al. they found that daptomycin on exposure to S. aureus in vitro resulted in decreased inflammatory responses [23].
The aim of current research was to assess the effect of daptomycin in an rat model of stroke, in the experiment daptomycin was administered at very early stage of stroke when MMPs lead to opening of BBB and neuroinflammation [9,24-26]. The study was guided by MRI, histological studies and evaluation of biochemical parameters throughout to monitor the modeling due to therapy of daptomycin against the induced stroke followed by study involving expression of cytokines.
Materials and Methods
Model for middle cerebral artery occlusion (MCAO) along with reperfusion
The experiments were sanctioned by Institutional ethical committee, China. For the study 60 rats which were Spontaneously Hypertensive (SHR) weighing between 275-300 g weight was chosen (Harlan, USA). The rats were housed in polypropylene cages, 12 h-12 h day and night light cycle and temperature was maintained at 24 ± 2°C. All the animals were given recommended diet with potable tap water ad libitum. The rats were subjected to 1.5 h of MCAO followed by 24 h and 48 h, 7 days, 14 days and 28 days of reperfusion [2]. To evaluate the effect of daptomycin the rats were divided in groups as daptomycin (n=15) and vehicle treated (n=15). In each treatment groups rats were perfuse till 4 weeks of reperfusion. A single dose (one time) of daptomycin (50 mg/kg) [27] and vehicle was given subcutaneously immediately after reperfusion onset through the right femoral vein. In the study a single dose of daptomycin was selected which was in accordance to previous studies [7,9] suggesting a single one time dose of MMP inhibitors before MCAO.
Magnetic resonance imaging (MRI)
Magnetic resonance imaging also called MRI of rats was done after 48 h, 1, 2 and 4 weeks post reperfusion. For the study rats were placed in MRI scanner using a holder, the animals were placed in the center of MRI scanner (4.7 Tesla, Bruker). Before the process of recording scans rats were subjected to anesthesia by mechanical ventilation using isoflurane (2.5%). During whole experiment the rates of respiration, heart rate were monitored with maintaining body temperature at 37 ± 0.5°C. The T2 scans were collected using a rapid spinecho sequence, T-2 images were delineated for recording infract and periinfract area where the images of delineated areas served as reference for other. For all the other protocols same location of slice was dictated. To assess tissue structure and organization multi-shot, multi-slice and diffusion-weighted Echo-Planar Imaging (EPI) Apparent Diffusion Coefficient (ADC) and Fractional Anisotropy (FA) maps were evaluated on base of voxel-wise basis using linear least-squares fit for the intensity of signal against the b-value for every diffusion direction. In the experiment the maps for ADC and FA were formed opting software ParaVision 5.1.
Cerebral Blood Flow (CBF) in rats was recorded opting Arterial Spin Labeling method (ASL). For ASL parameters the order consisted of FAIR-RARE. Evaluation of BBB permeability, dynamic contrast enhanced (DCE)-MRI was done using T1 mapping which included scans of pre-contrast (3 sequence) and post-contrast with 16 sequence upto 45 minutes. For the same a contrast producing agent Gd-DTPA was injected into the femoral vein at a dose of 0.1 mM/kg.
Histological studies
For the study six rats from each group were subjected to anesthesia followed by fixing with a PBS 4% opting transcardial perfusion. At the same time the rats were operated and brain was removed and embedded using paraffin for cutting into slices of 5 μm. The tissue slices were subjected to staining by standard hematoxylin and eosin dye followed by observation under light microscope for notable changes.
Immunohistochemistry studies
The expression of TNF-α and NF-κB was done by assessing the prepared brain tissue slices of 5 μm. Briefly, the sections of tissue were dewaxed and subjected to boling in citrate buffer at 95°C (approx) for time of 15 min to retrieve antigen. The sections then were followed by incubation along with antibodies (Iry) for time interval of 120 min and then incubation in presence of H2O2 (3%) and blocking by goat serum. After this the slices were washed with PBS followed by incubation with rabbit polyclonal overnight at 4°C (anti-TNF- α, 1:5). The sections were then subjected to incubation along with biotinylated Iry antibody followed by washing and incubation with horseradish streptavidin (peroxidase-labeled). At last after the development of slides by exposing them to diaminobenzidine followed by counterstaining with hematoxylin. Negative control was devoid of Iry antibody.
Capturing of images was done by Leica microscope (Leica Microsystems at X400), the slides were selected randomly and average cells found to be positive in each selected zone were calculated using cell image analysis management system (Image-Pro PlusUSA).
Western blots
Expression of proteins was assessed in white matter subjected to ischemia. Proteins were extracted from ischemic and nonischemic zone of brain in buffer for Radio Immune Precipitation Assay (RIPA). Polyvinylidene membranes (PVDF) were used and were transferred with proteins and were then incubated with Iry antibodies such as occluding (1:5), cludin-5 (1:5), zona occluden-1 (1:5), TNF-α (1:5), MMP-3 (1:1000), IL10 (1:1000), IL-1β (1:5), TGF- β (1:5), YM1 (1:4). The blots were recorded after the membranes were incubated with IIry antibodies; actin was used as loading control. The results obtained are presented as band intensity for expression of proteins.
Gelatin zymography
Gelatin zymography was utilized to analyse MMP-2 and MMP-9 from brain tissue proteins. The procedure briefly, a 50 μg of protein was separated by electrophoresis using a 10% SDS polyacrylamide gel. The gels after electrophoresis were subjected for incubation of 90 h, maintaining temperature of 37°C in presence of a developing buffer, the gels were subsequently subjected to staining with Brilliant blue R for 30 mins. Distaining of gels was done to obtain clear bands for studying gelatinolytic activity against a blue background, densitometry studies of gels was done. As standards for MMP-2 and MMP-9 supernatants from fibrosarcoma of humans cells was utilized.
Statistical evaluation of data
To compare two groups unpaired t-tests/Analysis of Variance (ANOVA) was performed. All the data are presented as ± SE. In all the group studies for comparison the data was considered significant when p<0.05. The data was analysed using graph pad software, USA.
Results
Protective effect of daptomycin against outcome of stroke in recovery phase
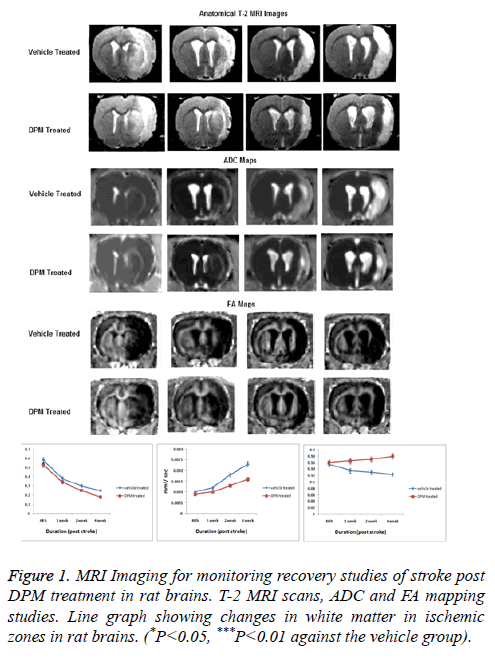
By non-surgical intervention of MRI the size of lesions were measured in the ischemic zone of brain (Figure 1). The study demonstrated significant results in T-2 weighted scans of DPM treated rats compared to vehicle treated. The scans of DPM treated rats showed smaller lesions after 2 weeks till the 4th week. The ischemic hemisphere showed decreased diffusivity in damaged tissues with MRI scans showing decreased ADC values after 2 and 4 weeks (Figure 1). However in DPM treated rats the extent of tissue breakdown was low versus the vehicle treated group after the 2 and 4 weeks of stroke. The FA in DPM treated rat’s established protective effect of treatment on white matter after 4 weeks.
Daptomycin increases cerebral blood flow (CBF) and decreases the permeability of blood–brain barrier in peri-infarct areas of brain
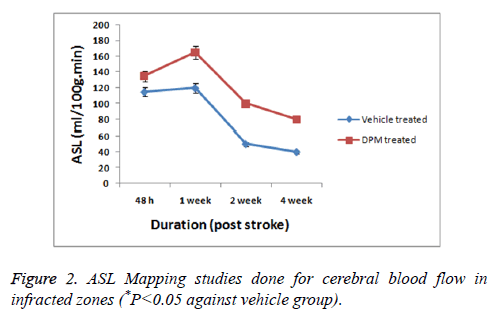
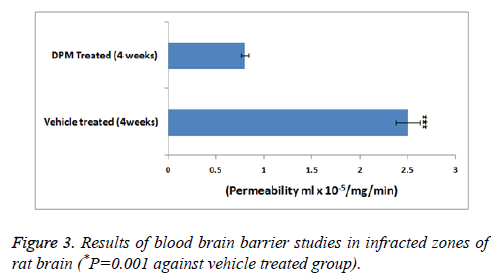
As a measure of cerebral blood flow in the experiment ASL was measured (Figure 2). The CBF mapping demonstrated higher perfusion in zones showing lesions chiefly in the areas surrounding the infracted zones after 2 days and 1 week of stroke versus the normal zones of brain observed previous studies [17]. The studies further showed that the perfusion in zones with lesions progressively decreased with time i.e. after 2 weeks, no changes in perfusion was seen in periinfarct zones. The permeability studies of BBB as shown by DCE-MRI demonstrated DPM lead to decreased values of (Ki) in the periinfarcted zones at 4 weeks versus the vehicle treated group (Figure 3).
Western blot analysis and effect of daptomycin on levels of occluding junction’s proteins and matrix metalloproteinase
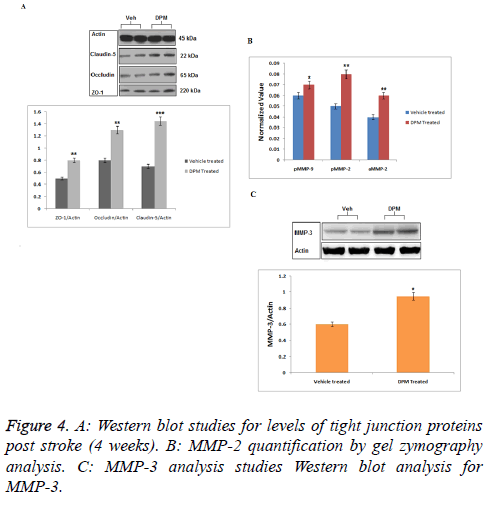
Western blot analysis was done to evaluate effect of DPM on expression of Occluding Proteins (OLP) such as occluding, zona occluding-1, claudin-5. Results of western blot analysis suggested higher levels of all three proteins in ischemic area in brains of DPM treated rats vs. vehicle fed rats (Figure 4A). Results suggested (Figure 4B) significant increased expression of both active and pro MMP-2 in both the groups (vehicle and DPM-treated). The rats with ischemic brains showed expression MMP-9 towards lower side while the levels of MMP-3 were found to increase in both the groups treated (vehicle and DPM treated) (Figure 4C). The study also marked that DPM do not produced any significant effect on the levels of TLP and MMP in tissues.
Immunohistochemistry and levels of inflammatory cytokines
Histological analysis of brain was done by staining with hematoxylin and eosin staining. The vehicle treated rats showed tissues with larger infracted area with disordered tissue arrangements. The brain tissue slides showed elevated cytoplasm volume in form of pale staining and hypervacuolization. The rats treated with DPM showed decreased cytoplasmic hypervacuolization the results were in agreement with MRI scans.
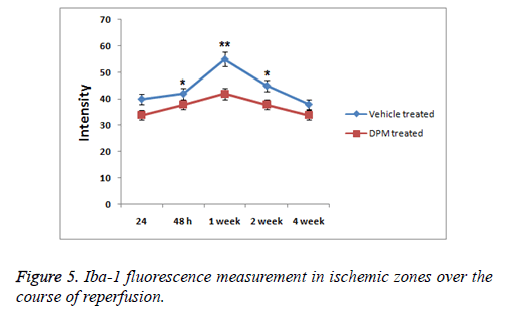
Inflammatory response was measured to evaluate any possible role of DPM in repairing brain injury, expression of proteins NF κB, TNF α, IL 10, IL 6 and IL 1β was analysed. Results of study showed levels of expression of IL 1β and IL 10 increased significantly in rat serum with stroke. DPM treated rats showed decreased expression of IL-10 and IL 1β versus the vehicle treated groups (p<0.05). Levels of IL-6 increased with DPM treatment. Immunohistochemistry studies were done to evaluate TNF α levels and NF κB in the brain. Expression of subunits NF κB p65 and TNF α increased in vehicle treated group while the expression levels decreased in rats after DPM treatment (Figure 5). The findings suggested DPM attenuated the extent of inflammation by regulating the mediators of inflammation (TNF α, IL 1β, IL 10 and IL 6) and NF κB versus the ischemic injury.
Discussion
In the current research we evaluated protective effect of Daptomycin after a single dose in early stage stroke subjected to reperfusion. Study was supported by non-invasive technique of MRI. The study established effective role of DPM in remodeling and recovering brain damage after stroke. DPM demonstrated remodeling of tissues in the peri-infracted zones by encouraging formation of TJP, the onetime dose also prompted the function of BBB during the process of recovery. The single dose of DPM did not affect the expression levels of MMP-2 and MMP-3 which are the very important enzymes required for angiogenesis during stroke in the course of recovery. DPM corrected inflammatory response in brain tissue which was confirmed by evaluating expression levels inflammatory mediators.
Daptomycin has been found to decrease production of cytokinins [22] also it has been evidenced to reduce inflammatory response [23]. DPM has also shown to interfere with MMPs [20] and due to its high penetrating property to cross BBB it is also a drug of choice in acute bacterial brain meningitis [20]. No studies are till date reported involving role of DPM followed by stroke in early stages involving treatment regimens of reperfusion.
Results of MRI involved in studying the anatomy of brain by T2, ADC and FA showed protective effect of DPM against brain injury in rats after 2 and 4 weeks followed by stroke. It can be postulated that onetime dose of DPM may exert neuroprotective action on BBB by reducing the involved IIry injury which may be characterized by release and infiltration of mediators of inflammation, infiltration of neurotoxic blood components and formation of MMPs [28,29], all these factors can be considered for recovery from stroke.
The T-2 weighted MRI scans revealed smaller lesions in rats who received DPM dose compared to vehicle treated post 2 and 4 weeks of stroke, also the extent of tissue break down was low in DPM treated rats and results of FA suggested beneficial effect of DPM on white matter in brain of rats subjected to stroke after 4 weeks.
ASL data analysis studies for mapping CBF showed elevated flow in peri-infract region of DPM treated rats progressively from 1st to 4th week followed by perfusion. The permeability coefficient maps (Ki) showed decreased permeability to BBB in rats treated with DPM after the 4th week of stroke which was in association with ASL values indicating increased blood flow. Parallel to results of MRI the western blot analysis suggested treatment of DPM increased the expression of protein such as claudin-5, occludin and also tight junction protein ZO-1.
Possible role of DPM in inflammatory mechanism was also evaluated. Inflammation has been accounted for pathogenesis of brain injury [30]. The cytokines chiefly related to inflammation associated stroke are TGF β, Tumor necrosis factor (TNF α) the Interlukins IL 1, IL 6, IL 10 which are found to be over expressed in case of brain injury [31]. Results of present study concluded that stroke resulted in elevated expression of interlukin IL 1β and tumor necrosis factor TNF α in vehicle treated rats and caused decrease in expression levels of IL 1β and TNF α in DPM treated rats. IL-6 one of the pro inflammatory cytokine was found up regulated after DPM treatment. IL-10 also called as the anti-inflammatory cytokine works by inhibiting interlukin (IL-1) and tumor necrosis factor (TNF-α), results revealed increased serum expression levels of IL-10 of stroke induced and vehicle treated rats and found to decrease in DPM treated rats.
The diametric transcription factor NF-κB is found to be associated in regulation of inflammation which is correlated to increased levels of IL 1β and also TNF α after stroke along with reperfusion [32,33]. In current research stroke markedly caused increased levels of NF κB (p65) in tissues of brain and also increased in levels of TNF α in DPM decreased the response.
Conclusions
In the present work we established that a single unit dose of daptomycin given very early stage after stroke provides a protective effect to neuronal tissues. We evidenced that DPM reduced damage of BBB and also protected blood vessels in damaged neuronal zones in very acute stage. This protective role of BBB led to increased perfusion and protected the tissues from reperfusion injuries. DPM also reduced inflammation which was evidenced in histochemical analysis. As strokes providing limited time for correcting acute phase injuries, the promising role of therapy consisting Daptomycin accompanied by non-surgical interventional therapies like MRI will impart valuable preclinical evidences which would definitely benefit the clinical outcomes.
Authors Contributions
Chunshui Yang designed the research protocol and laboratory studies. All the data were recorded and analysed by him and prepared the manuscript. Jianqing Pan and Chuanming Wang assisted in animal protocols and in preparation of manuscript with calculations. Wenfei li and Lijie Ren carried and explained the literatures to the team. All authors have read and approved the final manuscript.
Conflict of Interest statement
The authors have no conflict of interest.
Financial Support
The study was supported by the Shenzhen Foundation of Science and Technology (No: JCYJ 20140411094009914 and JCYJ 20150605103420338).
References
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 2003; 4: 399-415.
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet 2008; 371: 1612-1623.
- Muir KW, Tyrrell P, Sattar N, Warburton E. Inflammation and ischaemic stroke. Curr Opin Neurol 2007; 20: 334-342.
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 1999; 22: 391-397.
- Brouns R, De Deyn PP. The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg 2009; 111: 483-495.
- Yang Y, Candelario-Jalil E, Thompson JF, Cuadrado E, Estrada EY, Rosell A. Increased intranuclear matrix metalloproteinase activity in neurons interferes with oxidative DNA repair in focal cerebral ischemia. J Neurochem 2010; 112: 134-149.
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 2007; 27: 697-709.
- Yang Y, Thompson JF, Taheri S, Salayandia VM, McAvoy TA, Hill JW. Early inhibition of MMP activity in ischemic rat brain promotes expression of tight junction proteins and angiogenesis during recovery. J Cereb Blood Flow Metab 2013; 33: 1104-1114.
- Yang Y, Candelario-Jalil E, Thompson JF, Cuadrado E, Estrada EY, Rosell A. Increased intranuclear matrix metalloproteinase activity in neurons interferes with oxidative DNA repair in focal cerebral ischemia. J Neurochem 2010; 112: 134-149.
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 2007; 27: 697-709.
- Amantea D, Nappi G, Bernardi G, Bagetta G and Corasaniti MT. Post ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J 2009; 276: 13-26.
- Tuttolomondo A, Di Raimondo D, di Sciacca R, Pinto A, Licata G. Inflammatory cytokines in acute ischemic stroke. Curr Pharm Des 2008; 14: 3574-3589.
- Terao S, Yilmaz G, Stokes KY, Ishikawa M, Kawase T. Inflammatory and injury responses to ischemic stroke in obese mice. Stroke 2008; 39: 943-950.
- Han HS, Yenari MA. Cellular targets of brain inflammation in stroke. Curr Opin Investig Drugs 2003; 4: 522-529.
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci 2001; 2: 734-744.
- Qing W, Xian NT, Midori AY. The inflammatory response in stroke. J Neuroimmuno 2007; l184: 53-68.
- Yang GY, Gong C, Qin Z, Ye W, Mao Y, Bertz AL. Inhibition of TNFalpha attenuates infarct volume and ICAM 1 expression in ischemic mouse brain. Neuro Report 1998; 9: 2131-2134.
- Guo RB, Wang GF, Zhao AP, Gu J, Sun XL, Hu G. Paeoniflorin protects against ischemia induced brain damages in rats via inhibiting MAPKs/NF-κB mediated inflammatory responses. PLoS One 2012; 7: 49701.
- Tarkowski E, Rosengren L, Blomstrand C, Wikkelso C, Jensen C, Ekholm S, Tarkowski A. Early intrathecal production of interleukin 6 predicts the size of brain lesion in stroke. Stroke 1995; 26: 1393-1398.
- Grandgirard D, Schurch C, Cottagnoud P, Leib SL. Prevention of brain injury by the nonbacteriolytic antibiotic daptomycin in experimental pneumococcal meningitis. Antimicrob. Agents Chemother 2007; 51: 2173-2178.
- Kelesidis T. The interplay between daptomycin and the immune system. Front Immunol 2014; 5: 52.
- Pichereau S, Moran JJ, Hayney MS, Shukla SK, Sakoulas G, Rose WE. Concentration-dependent effects of antimicrobials on Staphylococcus aureus toxin-mediated cytokine production from peripheral blood mononuclear cells. J Antimicrob Chemother 2012; 67: 123-910.
- English BK, Maryniw EM, Talati AJ, Meals EA. Diminished macrophage inflammatory response to Staphylococcus aureus isolates exposed to daptomycin versus vancomycin or oxacillin. Antimicrob Agents Chemother 2006; 50: 2225-2710.
- Yang Y, Candelario-Jalil E, Thompson JF, Cuadrado E, Estrada EY, Rosell A. Increased intranuclear matrix metalloproteinase activity in neurons interferes with oxidative DNA repair in focal cerebral ischemia. J Neurochem 2010; 112: 134-149.
- Fagan SC, Waller JL, Nichols FT, Edwards DJ, Pettigrew LC, Clark WM. Minocycline to improve neurologic outcome in stroke (MINOS): a dose finding study. Stroke 2010; 41: 2283-2287.
- Yang Y, Hill JW, Rosenberg GA. Multiple roles of metalloproteinases in neurological disorders. Prog Mol Biol Transl Sci 2011; 99: 241-263.
- Xu L, Fagan SC, Waller JL, Edwards D, Borlongan CV, Zheng J. Low dose intravenous minocycline is neuroprotective after middle cerebral artery occlusion-reperfusion in rats. BMC Neurol 2004; 4: 7.
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci 2008; 28: 9451-9462.
- Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 2008; 39: 1121-1126.
- Muir KW, Tyrrell P, Sattar N, Warburton E. Inflammation and ischaemic stroke. Curr Opin Neurol 2007; 20: 334-342.
- Han HS, Yenari MA. Cellular targets of brain inflammation in stroke. Curr Opin Investig Drugs 2003; 4: 522-529.
- Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol 1994; 12: 141-179.
- Berti R, Williams AJ, Moffett JR. Quantitative real time RT PCR analysis of inflammatory gene expression associated with ischemia reperfusion brain injury. J Cereb Blood Flow Metab 2002; 22: 1068-1079.