Image Article - Case Reports in Surgery and Invasive Procedures (2023) Volume 7, Issue 3
Dank and Alfresco-Extraskeletal Myxoid Chondrosarcoma
Anubha Bajaj*Department of Pathology, Panjab University, Chandigarh, India
- *Corresponding Author:
- Anubha Bajaj
Department of Pathology
Panjab University, Chandigarh, India
E-mail: anubha.bajaj@yahoo.com
Received: 03-May-2023, Manuscript No. AACRSIP-23-10030; Editor assigned: 06-May-2023, PreQC No. AACRSIP-23-10030(PQ); Reviewed: 20-May-2023, QC No. AACRSIP-23-10030; Revised: 24-May-2023, Manuscript No. AACRSIP-23-10030(R); Published: 31-May-2023, DOI:10.35841/aacrsip-7.3.150
Citation: Bajaj A. Dank and alfresco-extraskeletal myxoid chondrosarcoma. Case Rep Surg Invasive Proced. 2023;7(3):150
Abstract
Extraskeletal myxoid chondrosarcoma denominates an exceptionally discerned, malignant, mesenchymal neoplasm of uncertain differentiation. Previously designated as NR4A3 rearranged myxoid sarcoma, the multi-lobulated tumefaction is comprised of uniform tumour cells configuring cords, clusters or reticular networks enmeshed within an abundantly myxoid matrix. Characteristically, neoplastic cells appear interconnected and articulate miniature cords, clusters, complex trabecular articulations or cribriform arrays. Neoplasm is composed of bland tumour cells incorporated with eosinophilic cytoplasm, spherical or elliptical nuclei, uniform nuclear chromatin and inconspicuous nucleoli. However, focal cartilaginous differentiation is absent. Tumour cells consistently display NR4A3 genetic rearrangement. The infrequently encountered extraskeletal myxoid chondrosarcoma configures <1% of soft tissue sarcomas. Generally, tumefaction incriminates adult subjects and is preponderant within 4th decade to 7th decade. A male predominance is encountered with male to female proportion of ~2:1 [1,2].
Majority of neoplasms arise within deep seated soft tissue of proximal extremities and limb girdles wherein the thigh is frequently incriminated. Infrequently, neoplasm may emerge within pelvis, masticator space, bone, pulmonary parenchyma, dura, retroperitoneum or pleura [1,2]. Of obscure aetiology, extraskeletal myxoid chondrosarcoma is preponderantly driven by various genetic fusions. Characteristically, extraskeletal myxoid chondrosarcoma exhibits genetic rearrangement of NR4A3 gene which is pre-eminently fused with EWSR1 gene. Uncommonly, diverse partner genes as TAF15, TCF12, TFG, FUS or HSPA8 may demonstrate aforesaid genomic fusion [2,3].
Chromosomal translocation t(9;22)(q22;q12) or infrequently, translocation t(9;17)(q22;q11) or t(9;15)(q22;q21) may be enunciated. Extraskeletal myxoid chondrosarcoma represents as a gradually progressive, minimally painful soft tissue tumefaction. Nearly 87% of incriminated subjects manifest with primary, localized disease whereas 13% subjects enunciate distant metastases. Majority (80%) of neoplasms demonstrate initial or preliminary distant metastasis within pulmonary parenchyma. Upon gross examination, a well demarcated, lobulated neoplasm is encountered wherein myxoid nodules appear segregated by fibrous tissue septa. Focal cystic areas and haemorrhage may be discerned. Upon frozen section, uniform cells permeated with eosinophilic cytoplasm and spherical or spindle shaped nuclei configure cords or clusters which are embedded within an abundantly myxoid matrix. Cytological evaluation enunciates hypocellular or hyper-cellular smears constituted of elliptical or polygonal tumour cells articulating clusters, trabeculae or cellular cords.
Neoplastic cells are permeated with eosinophilic, finely vacuolated cytoplasm and bland, spherical to indented nuclei with uniform dissemination of nuclear chromatin, nucleoli of variable magnitude, nuclear inclusions or nuclear grooving. Tumour cell magnitude and nuclear dimensions appear monotonous and regular. Foci of rhabdoid cellular differentiation may be encountered. Circumscribing myxoid stroma is abundant [2,3]. Upon microscopy, neoplasm exhibits a multinodular architecture wherein fibrous tissue septa traverse the tumour parenchyma and demarcate neoplastic cells from encompassing pools of abundant, myxoid or chondromyxoid matrix [3,4].
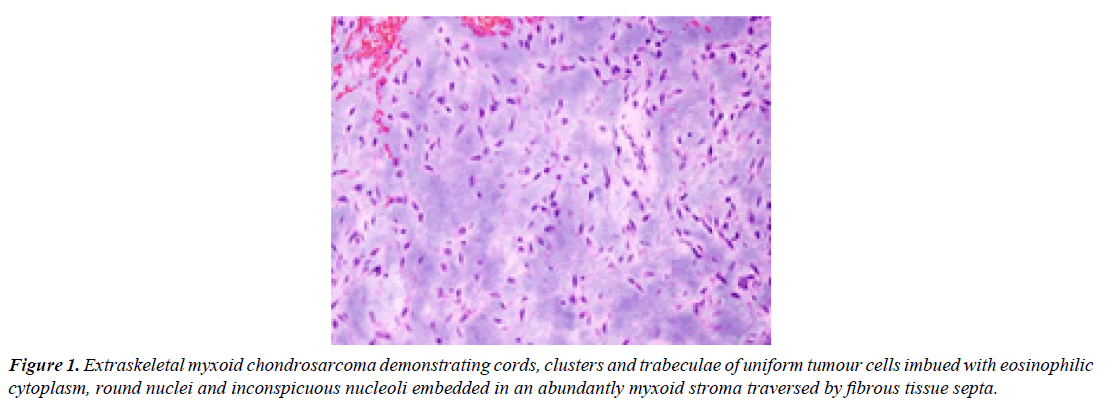
Tumour cells are uniform and permeated with eosinophilic to vacuolated cytoplasm with elongated, delicate cytoplasmic processes, spherical to elliptical nuclei, evenly dispersed nuclear chromatin and inconspicuous nucleoli. Characteristically, tumour cells appear interconnected and articulate cords, miniature clusters, complex trabeculae or cribriform arrays. Differentiation into spindle shaped cells is commonly encountered. Surrounding stroma is hypovascular. Mitotic activity is minimal. Miniature or enlarged foci of intralesional haemorrhage are commonly exemplified. Generally, foci of cartilaginous differentiation are absent. Majority (80%) of neoplasms demonstrating variant (non- EWSR1) NR4A3 genetic fusion with TAF15 or TCF12 genes manifest as high grade lesions with increased cellularity, cellular proliferation and cytological atypia wherein ~50% of aforesaid neoplasms exhibit epithelioid cells or rhabdoid features. Upon ultrastructural examination, distinct cords of tumour cells appear immersed within a matrix with abundant glycosaminoglycan. Membrane bound dense core granules may be discerned, akin to neurosecretory granules. Tumour cells delineate abundant mitochondria and well developed Golgi apparatus. Intracysternal microtubules may be exemplified [3,4] (Figure 1).
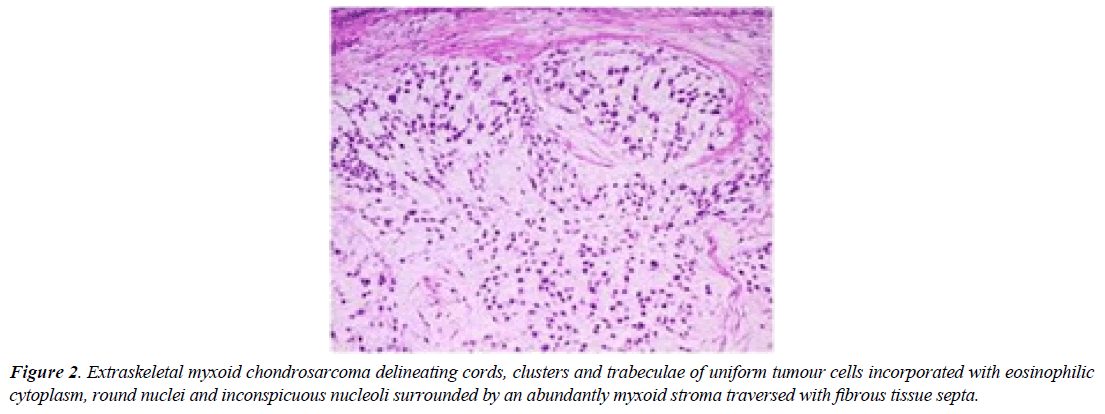
Extraskeletal myxoid chondrosarcoma is immune reactive to Neuron Specific Enolase (NSE), synaptophysin, S100 protein or insulinoma associated protein 1(INSM1). A subset of neoplasms appear immune reactive to INSM1 as chordoma, soft tissue myoepithelioma, ossifying fibromyxoid tumour and Ewing’s sarcoma (Figure 2).
Extraskeletal myxoid chondrosarcoma is immune nonreactive to epithelial markers as AE1/AE3, CAM5.2 or Epithelial Membrane Antigen (EMA). Extraskeletal myxoid chondrosarcoma requires segregation from neoplasms such as soft tissue myoepithelioma, ossifying fibromyxoid tumour, soft tissue chordoma, epithelioid variant of myxofibrosarcoma or myxoid leiomyosarcoma. Extraskeletal myxoid chondrosarcoma may be appropriately discerned with cogent tissue sampling and categorization of characteristic genetic rearrangement within NR4A3 gene. Upon Computerized Tomography (CT) and Magnetic Resonance Imaging (MRI), a lobulated tumefaction is encountered. Upon T1 weighted imaging, tumefaction exhibits decimated signal intensity. Upon T2 weighted imaging, neoplasm enunciates enhanced signal intensity. Following administration of gadolinium contrast, tumour depicts enhancing intrinsic fibrous tissue septa traversing the neoplasm. Optimal, recommended mode of therapy for treating localized extraskeletal myxoid chondrosarcoma is surgical eradication of neoplasm. Adjuvant radiation therapy may or may not be adopted. Although prolonged survival may be expected, proportionate tumour relapse occurs in ~50% subjects. Advanced neoplasms are treated with contemporary, standardized chemotherapy applicable to soft tissue sarcoma [4,5].
Besides, employment of antiangiogenic agents may be beneficial. Extraskeletal myxoid sarcoma demonstrates a prolonged clinical course. 5 year survival appears at ~90%, 10 year survival emerges at ~70% and 15 year survival manifests at ~60%. Inferior prognostic outcomes are encountered within elderly incriminated subjects, enlarged neoplasms >10 centimetre magnitude or proximal location of neoplasm. Decimated metastasis free or overall survival ensues with emergence of features as enhanced tumour cellularity, occurrence of cellular and nuclear anaplasia or rhabdoid features, mitotic activity >2 mitosis per 10 high power fields, Ki67 ≥ 10% and Ki67 hot spot ≥ 25%.
In contrast to tumefaction exemplifying EWSR1-NR4A3 genetic fusion, neoplasms delineating variant non-EWSR1 genetic fusion demonstrate enhanced incidence of rhabdoid cells, tumours of advanced grade and aggressive biological behaviour with inferior prognostic outcomes [4,5].
References
- Fice MP, Lee L, Kottamasu P, et al. Extraskeletal myxoid chondrosarcoma: A case series and review of the literature. Rare Tumors. 2022;14:20363613221079754.
- Brown JM, Rakoczy K, Pretell-Mazzini J. Extraskeletal myxoid chondrosarcoma: Clinical features and overall survival. Cancer Treat Res Commun. 2022;31:100530.
- Omote M, Tsubamoto H, Ide Y, et al. Extraskeletal myxoid chondrosarcoma of the vulva: A case report. Cureus. 2023;15(1).
- Zhu ZY, Wang YB, Li HY, et al. Primary intracranial extraskeletal myxoid chondrosarcoma: A case report and review of literature. World J Clin Cases. 2022;10(13):4301.
- Xia H, Liu G, Wu A, et al. Intra-muscular extraskeletal myxoid chondrosarcoma of the thigh: A case report. Int J Clin Exp Pathol. 2022;15(12):476.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref