- Biomedical Research (2016) Volume 27, Issue 3
Cytokine and immunoglobulin levels in patients undergoing supratentorial craniotomy.
Guo-Yan Li1, Yan-Bin Sun2, Xiaoning Li3, Xia Li1, Li-Zhi Sun1, Fang-Cai Lin3, Bao-Guo Wang4*1Department of Anesthesiology, North China Grid Company Limited Beijing Electric Power Hospital, Beijing, China
2Department of Anesthesiology, The Central Hospital of Chengde City, No.22, Guangren Street, Chengde City, Hebei Province, China
3The second Department of Anaethesiology, No. 1 Hospital of Shijiazhuang, China
4Department of General Surgery, North China Grid Company Limited Beijing Electric Power Hospital, Beijing, China
- *Corresponding Author:
- Bao-Guo Wang
Department of Anesthesiology and Pain Management
Capital Medical University
China
Accepted date: February 16, 2016
Abstract
Introduction: Surgery could directly cause an inflammatory response and stimulate the release of cytokines, such as interleukin (IL)-8, tumor necrosis factor-α (TNF-α) and IL-10. Infection and postoperative complications are higher risk factors after craniotomy, and the impairment of immune function is associated with post infection and complications. The aim of our study was to investigate the changings of cytokines and immunoglobulins levels in patients received craniotomy surgery.
Methods: A total of 18 patients undergoing craniotomy were studied. Blood samples were collected before anesthesia (T0), 30 min(T1), 2h(T2), 4h(T3) after induction of anesthesia, and 1 day post-surgery (T4), 2 days post-surgery (T5); the levels of tumor necrosis factor-α (TNF-α), interleukin (IL)-8, IL-10, IgM, IgA, IgG were measured. Data were analyzed by SPSS 13.0 software using repeated-measures analysis of variance followed by a Bonferroni correction.
Results: Compared with T0, the peripheral blood IgA levels decreased significantly at T2, T3 and T4 time points. The peripheral blood IgM levels decreased significantly at T2 and T3 time points. IL-10 in peripheral blood increased significantly at T2, T3 and T4 compared with T0 time point. IL-8 in peripheral blood increased significantly at T3, T4 and T5 time point. The concentration of the TNF-α and IgG in peripheral blood did not change significantly.
Conclusions: Anaesthesia and surgery can cause pro-inflammatory response and anti- inflammatory response in patient undergoing craniotomy; the immune function was suppressed at the same time.
Keywords
Cytokines, Immunoglobulins, Supratentorial craniotomy.
Introduction
Postoperative infections are potentially fatal complications which common occurred in neurosurgical intensive care [1]; the occurrence of postoperative infections increase the risk of sepsis and multiple organ failure [2].
Pro-inflammatory response is a compensatory antiinflammatory response to attenuate the pro-inflammatory state [3], and the balance between the pro- and anti-inflammatory responses determines the net outcome of the inflammatory reaction, disequilibrium between pro- and anti-inflammatory cytokines may start a generalized response that in turn may progress to a multiple organ dysfunction [4].
The systemic inflammatory and immune parameters were adopted in many researches to quantify the degree of surgery and anesthesia-related trauma. Previous study revealed that immune cell decreased significantly after general anesthesia induction [5].
Anesthesia and surgery could induce inflammatory response, the triggering factors include surgical trauma, endotoxaemia and anesthetic, all of these factors induce activation of the transcription factors, including nuclear factor (NF)-κB, interleukins (IL), activation of NF-κB lead to release of IL-6, IL-8, IL-10, and tumour necrosis factor-alpha (TNF-α), the earlier inflammatory response markers. TNF-α, IL-8 and IL-10 are the key inflammatory mediators in central nervous system injury and play important role in the inflammatory response [6].
McBride et al. reported the cytokine response in patients undergoing cardiac surgery; they indicated the cytokine response is dominated by the pro-inflammatory cytokines TNF-α, IL-6, IL-8, and the anti-inflammatory cytokine IL-10. However, few report mentioned changes of pro-inflammatory cytokines and anti-inflammatory cytokines in craniotomy surgery, the aim of the study was to observe the inflammatory balance during and after craniotomy surgery.
Methods
Patients
patients receiving craniotomy surgery were included in our study. All the operations and medical examinations were conducted in Beijing Tiantan Hospital (Beijing, China). The study protocol was approved by the Institutional Review Board of the hospital and written informed consent was obtained from all patients. The ASA (American Society of Anesthesiologists) stages of these patients were grade I and II.
Patients with immune, renal or CNS dysfunction and those with congestive heart failure, exogenous hormone therapy (including steroids), pregnancy, malnutrition, diabetes, malignancy, infection or inflammation were excluded from the study. No analgesics or tranquilizers were administered to these patients before the operation.
Anaesthetic process
After entering the operation room, an intravenous infusion was given to the patient and non-invasive blood pressure (NIBP), heart rate (HR), oxygen pulse saturation (SPO2) and the bispectral index (BIS) were monitored. Demographic data of patient and details of the operation are shown in table 1.
| Gender (M/F) | 10/8 |
|---|---|
| Age (years) | 43±11 |
| Duration of surgery (min) | 249±45 |
| ASA physical status (I/II) | 14/4 |
| Tumor type (glioma/meningioma/other tumors) | 9/4/5 |
Table 1. Demographic data of these patients and their operative data.
Target concentration infusion anesthesia was performed using propofol and sufentanil. The induction plasma concentration of was 5 μg/ml (propofol) and 0.5 ng/ml (sufentanil) respectively. The plasma concentration of propofol was reduced to 3.2 μg/ml and sufentanyl was reduced to 0.3 ng/ml when the patients were unconscious, and vecuronium bromide 0.1 mg/kg was administered. After muscle relaxation, tracheal intubation was performed. Mechanical ventilation was applied with 10 ml/kg tidal volume, 12 times/min respiratory frequency and 1 L/min oxygen flow. Intermittent administration of 0.05 mg/kg vecuronium bromide was given to maintain muscle relaxation.
During this process, mean arterial pressure (MAP) and hear rate (HR) were maintained in the basic range of +10% to −20%; and MAP <20% of baseline for hypotension patient, HR<50 beats/min for bradycardia patient or MAP>10% of baseline values for hypertension; The 6 mg of ephedrine, 0.5 mg of atropine or 0.2–0.5 mg of nicardipine was administered respectively.
Sampling and measurement
3 ml of blood samples were taken in SSTII advance tubes (Becton Dickinson, UK) at T0 (before anesthesia), T1 (30 min after induction of anesthesia), T2 (2 h after induction), T3 (4 h after induction), T4 (1d after operation) and T5 (2d after operation) time points for measurement of cytokine and immunoglobulin concentrations. The cytometric bead assay kit (Becton Dickinson, USA) was used to measure levels of TNF- α, IL-8, IL-10, IL-2, IL-3, IFN-γ, IgM, IgA and IgG in serum according to described protocols (http://www.bdbiosciences.com/documents/CBA_BDFACSArray_Bioanalyzer_Kit_Manual.pdf).
Statistical analysis
Cytokine and immunoglobulin concentrations are presented as means ± SD and analyzed using SPSS 13.0 statistical software. Data were compared using the t test and Chi square test, respectively. A p value <0.05 was considered as significant. The Maunchly test was used to judge whether there were relations between the repeatedly measured data. When p<0.05, the Greenhouse–Geisser correction was used to correct the results.
Results
A total of 18 consecutive patients were included in this study, Demographic data of these patients and their operative data are listed in table 1. No operative morbidity or mortality occurred in observation period.
Cytokine concentration changes
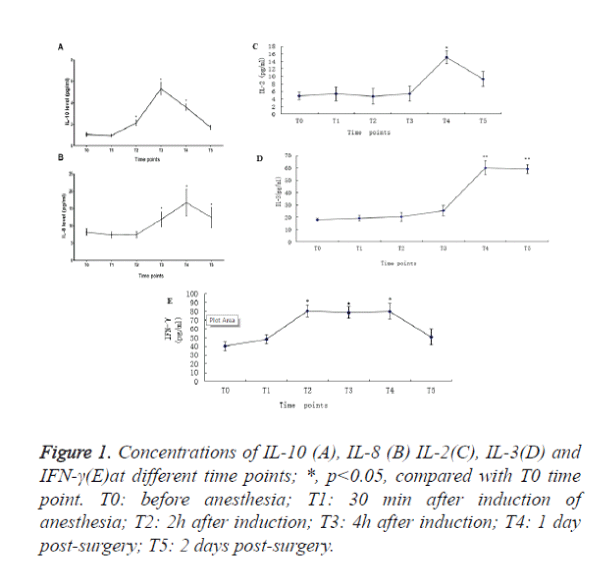
We observed that the IL-10 in peripheral blood increased 93.3%, 420% and 282% at T2, T3 and T4 time points respectively when compared with T0 time point (Figure 1A).
The IL-8 in peripheral blood increased 29.9%, 89.0% and 33.6% at T3, T4 and T5time points respectively when compared with T0 time point (Figure 1B). IL-2 (Figure 1C) and IL3 (Figure 1D) increased to peak at T4. IFN-γincreased from T2 and sustained to T4 (Figure 1E). There was no significant change in the level of TNF-α, (Table 2).
Figure 1. Concentrations of IL-10 (A), IL-8 (B) IL-2(C), IL-3(D) and IFN-γ(E)at different time points; *, p<0.05, compared with T0 time point. T0: before anesthesia; T1: 30 min after induction of anesthesia; T2: 2h after induction; T3: 4h after induction; T4: 1 day post-surgery; T5: 2 days post-surgery.
| To | T1 | T2 | T3 | T4 | T5 | |
|---|---|---|---|---|---|---|
| TNF-α (pg/ml) | 1.47±0.13 | 1.66±0.13 | 1.54±0.12 | 1.67±0.14 | 1.61±0.18 | 1.40±0.46 |
| IgG (g/l) | 0.50±0.05 | 0.56±0.06 | 0.52±0.06 | 0.51±0.05 | 0.44±0.08 | 0.45±0.07 |
Table 2. TNF-α and IgG levels in peripheral blood at different time point.
Immunoglobulin concentration changes
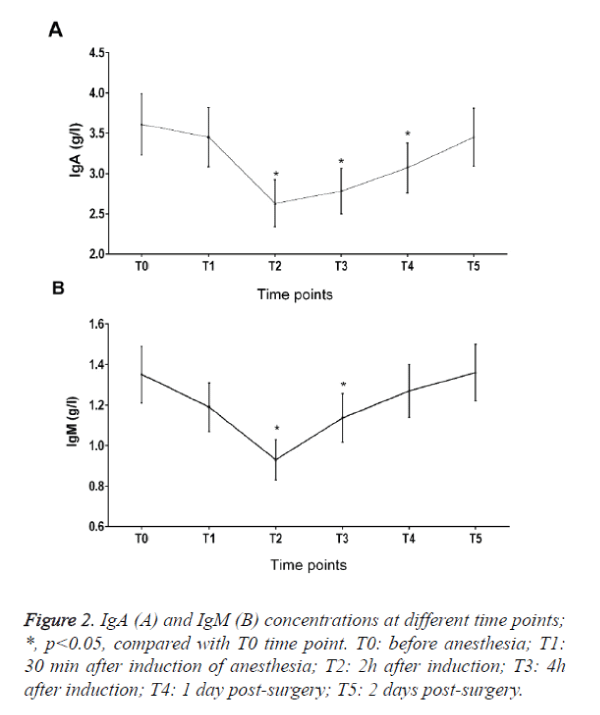
IgA in peripheral blood decreased 28.2%, 23.7% and 19.6% at T2, T3 and T4 time points when compared with T0 (Figure 2A). IgM in peripheral blood decreased 28.2% and 20.6% at T2 and T3 time points when compared with T0. There was also no significant change in the level of IgG (Figure 2B and Table 2).
Discussions
Surgery and related stress response lead to the suppression of immune function [7]. In this study, we found that IgM and IgA decreased significantly during perioperative period. In addition, a significant alteration in the levels of IL-10 and IL-8 was observed during perioperative period in patients undergoing supratentorial craniotomy. Inflammatory cells contribute to the clearance and the repairing of necrotic tissue, and dominate the local response to injury [8]. Inflammatory cells release soluble molecules, mainly include cytokines that act on distant sites from the origin of their production, while a systemic acute-phase response goes along the local inflammation, then followed by a compensatory antiinflammatory response to attenuate the proinflammatory state [3], and the balance between the pro- and anti-inflammatory responses determines the net outcome of the reaction. In major injury, disequilibrium between pro- and anti-inflammatory cytokines may start a generalized response that in turn may progress to a multiple organ dysfunction [4].
Research has revealed that excessive inflammatory responses might delay the recovery and attenuate hospital outcomes [9]. TNF-α, IL-6, IL-8 and IL-10 are well-known inflammatory cytokines; they could reflect the severity of inflammatory responses. Mei et al indicated that concentrations of plasma IL-6 and IL-8 might be able to evaluate the severity of inflammatory response and could be used for prognosis [10]. Cardiopulmonary bypass surgery (CPB) may cause inflammatory responses, Mei et al found plasma TNF-α, IL-6 and IL-8 levels increased significantly at the end of CPB and reach their peak 6 hour post-CPB, the IL-10 elevated and peaked at the end of CPB. In the present study, we found that IL-8 increased significantly 4 hour after induction of anesthesia and peaked 1 day post-surgery, which demonstrated that proinflammatory responses occurred. We also found that IL-10 increased significantly 2 hour after induction of anesthesia and peaked at 4 hour after induction, which also demonstrated that anti-inflammatory responses occurred.
There are five types of immunoglobulins in serum, IgA, IgD, IgE, IgM and IgG, the amounts of IgE and IgD are very small, so we only measured the levels of IgA, IgM and IgG. In our study, we found that IgM and IgA decreased significantly and reach their bottom 2 hour after induction, which suggested that the immune function was suppressed.
Liu et al found that at the 30min after anesthesia induction, the neutrophils, monocytes and lymphocytes during craniotomy decreased significantly [5], which was consistent with our study.
In conclusion, our study revealed that anesthesia and surgery can cause pro-inflammatory response and anti- inflammatory response in patient undergoing craniotomy, and the immune function was suppressed at the same time. However, more studies are still needed to investigate how to alleviate the immune suppression.
References
- Sablotzki A, Ebel H, Muhling J, Dehne MG, Nopens H, GiesselmannH, Hempelmann G. Dysregulation of immune response following neurosurgical operations. ActaAnaesthesiolScand 2000; 44: 82-87.
- Wakefield CH, Carey PD, Foulds S, Monson JR,Guillou PJ. Changes in major histocompatibility complex class II expression in monocytes and T cells of patients developing infection after surgery. Br J Surg 1993; 80: 205-209.
- Mannick JA, Rodrick ML,Lederer JA. The immunologic response to injury. J Am CollSurg 2001; 193: 237-244.
- Hranjec T, Swenson BR, Dossett LA, Metzger R, Flohr TR, Popovsky KA, Bonatti HJ, May AK, Sawyer RG. Diagnosis-dependent relationships between cytokine levels and survival in patients admitted for surgical critical care. J Am CollSurg 2010; 210: 833-836.
- Liu S, Wang B, Li S, Zhou Y, An L, Wang Y, Lv H, Zhang G, Fang F, Liu Z, Han R, Jiang T, Kang X. Immune cell populations decrease during craniotomy under general anesthesia. AnesthAnalg 2011; 113: 572-577.
- Hasturk A, Atalay B, Calisaneller T, Ozdemir O, Oruckaptan H, Altinors N. Analysis of serum pro-inflammatory cytokine levels after rat spinal cord ischemia/reperfusion injury and correlation with tissue damage. Turk Neurosurg 2009; 19: 353-359.
- Yokoyama M, Itano Y, Mizobuchi S, Nakatsuka H, Kaku R, Takashima T, Hirakawa M. The effects of epidural block on the distribution of lymphocyte subsets and natural-killer cell activity in patients with and without pain. AnesthAnalg 2001; 92: 463-469.
- Tidball JG. Inflammatory processes in muscle injury and repair. Am J PhysiolRegulIntegr Comp Physiol 2005; 288: R345-353.
- Onorati F, Rubino AS, Nucera S, Foti D, Sica V, Santini F, Gulletta E, Renzulli A. Off-pump coronary artery bypass surgery versus standard linear or pulsatile cardiopulmonary bypass: endothelial activation and inflammatory response. Eur J CardiothoracSurg 2010; 37: 897-904.
- Mei YQ, Ji Q, Liu H, Wang X, Feng J, Long C, Cheng B, Xing Y, Li J, Hu D. Study on the relationship of APACHE III and levels of cytokines in patients with systemic inflammatory response syndrome after coronary artery bypass grafting. Biol Pharm Bull 2007; 30: 410-414.