Research Article - Biomedical Research (2018) Volume 29, Issue 7
CXCR4 antagonist AMD3100 protects TGFβ1 induced viability and migration in rat cardiac fibroblasts
Ziqian He1,2, Xueting Wang2, Yi Zhou2, Zheng Lu2, Wanlan Chen2, Xiang Tang2, Huichao Pan2, Min Zhang2, Zhaohui Qiu1,2,3*1Huadong Hospital Affiliated with Fudan University, Shanghai, PR China
2Division of Cardiology, Tongren Hospital, School of Medicine, Shanghai Jiao Tong University, 1111 Xianxia Road, Shanghai, PR China
3Shanghai Key Laboratory of Clinical Geriatric Medicine, Fudan University, Shanghai, PR China
- *Corresponding Author:
- Zhaohui Qiu
Shanghai Key Laboratory of Clinical Geriatric Medicine
Fudan University
Shanghai, PR China
Accepted date: January 21, 2018
DOI: 10.4066/biomedicalresearch.29-18-269
Visit for more related articles at Biomedical ResearchAbstract
Proliferation and migration of Cardiac Fibroblasts (CFBs) are important in early stage of myocardial fibrosis. The purpose of the present study was to investigate the effect of CXCR4 antagonist AMD3100 treatment on transforming growth factor (TGF)β1induced CFBs abnormal proliferation and migration, and to elucidate the underlying mechanisms. The cell proliferation was evaluated by a MTT assay. Cell transwell migration assay and wound scratch assay were used to detect the cells migration. Measurement of ROS levels were determined by the DCF-DA dye. TGFβ1 stimulation in CFBs resulted in increased proliferation, migration and ROS generation. In conclusion, the present study revealed that AMD3100 treatment inhibited CFBs proliferation and migration induced by TGFβ1, at least in part through suppression of ROS signaling.
Keywords
CXCR4, AMD3100, TGFβ1, Cardiac fibroblasts, Proliferation, Migration.
Introduction
Cardiac fibroblast abnormal proliferation, migration and collagen proteins deposition in Extracellular Matrix (ECM) induces cardiac fibrosis, which is involved in myocardial tissue remodelling and dysfunction [1-3]. Cardiac tissue remodelling ultimately leads to elevated regional myocardial stiffness, left ventricular diastolic dysfunction, reduced the vascular resistance of the coronary arteries and sudden cardiac death [4-6].
Immune responses, ischemia-reperfusion injury and hyperglycemia induces cardiac fibrosis via activating the renninangiotensinaldosterone system, the collagen proteins generation and depredating cytokines production [7]. Thus, investigation of the pathogenesis and molecular mechanisms of cardiac fibrosis is necessary for the severe cardiovascular events prevention and treatment.
Transforming growth factor (TGF)β1 has been regarded as a multifunctional peptide [8]. TGFβ1 has a key role in various biological activities, especially in the cardiovascular system [9,10]. TGFβ1 modulates the transformed phenotype of fibroblasts and elevates the generation of collagen proteins and fibrosis [11-13].
Previous studies have also confirmed that TGFβ1 inhibits the activity of matrix metalloproteinase and inhibits ECM degradation in prostate cancer (PrCa) cells [14]. Specifically, TGFβ1 is involved in the modulation of multiple transduction pathways and directly stimulates the proliferation of CFBs [15,16].
More, TGF-β1 increased pancreatic carcinoma cells, human dermal fibroblasts and CFBs migration [17-19]. However, the molecular mechanisms underlying this abnormal alteration are poorly understood.
The previous studies demonstrated that the CXCR4 plays a key role in the pathogenesis of CFBs [20]. CXCR4 antagonist AMD3100 promoted wound healing in diabetic mice by increasing cytokine production and elevating the activity of fibroblasts [21].
Many studies found that TGF-β1 regulates CXCR4 expression in both mRNA and protein level, which then affected stem cell migration and adhesion [22,23]. However, whether TGF-β1 induces proliferation and migration of CFBs by CXCR4 remains unclear.
The aim of the present study was investigated whether treatment with AMD3100 could abolish the alterations in CFBs and ROS generation induced TGF-β1 and the molecular mechanisms that underlie the protective effects of AMD3100 on CFBs function with the suppression of the ROS pathway.
Materials and Methods
Materials
TGF-β1 was purchased from PeproTech, Inc. (Rocky Hill, NJ, USA). AMD3100 and Fetal Bovine Serum (FBS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fibroblast Medium-2 was from Sciencell (Shanghai, China). Dulbecco's modified Eagle's medium (DMEM) was purchased from Gibco (Thermo Scientific, Grand Island, NY, USA).
Cell culture
Rat cardiac fibroblasts were obtained from Sciencell (R6300, Shanghai, China). The cells were cultured in fibroblast medium-2 (2331, Sciencell, Shanghai, China) with 10% fetal bovine serum (FBS, Sigma-Aldrich, St. Louis, MO, USA) and 1% penicillin and streptomycin at 37°C incubator with 5% CO2. All experiments were carried out with cardiac fibroblast from 2-4 generation.
Cell treatment
CFBs were pre-treated with CXCR4 inhibitor AMD3100 (0-1 μg/ml), LY294002 (10 μM) for 30 min at 37°C and then added to TGF-β1 with different concentrations (0-20 ng/ml) for 24 h. The control group were added with PBS alone. After treatment, the cells were collected for further investigation.
Cell proliferation assay
Cells (5 × 103 cells/well) were treated with TGF-β1 and AMD3100 for 24 h. The cells were washed twice with PBS, and were incubated with 5 mg/ml MTT reagent (20 μl, Beyotime Technology) at 37°C for 4 h, the cells were dissolved in DMSO (150 μl, Sigma-Aldrich, Inc., USA). The absorbance was measured by a microplate reader (Bio-Rad, CA, USA) at 490 nm.
Cell migration assay
CFBs migration was determined by using transwell culture plates with 8.0 μm pore polycarbonate membrane (Corning Costar, Cambridge, MA, USA). CFBs (2 × 104 cells/well) was seeded to the upper insert, the lower chamber were added 500 μL of DMEM medium containing TGF-β1 with different concentrations (0-20 ng/ml) at 37°C in a humidified, 5% CO2 atmosphere for 12 h. Then, the DMEM medium was removed from the upper insert and non-migrated cells were removed by scraping. The migrated cells were stained with Calcein-AM at 37°C in the dark for 30 min. Migrated cells were quantified with a light microscope (200x magnification, Carl Zeiss Microimaging, Thornwood, NY, USA).
Wound scratch assay
CFBs (2 × 104 cells/well) were suspended in 6-well plates (Thermo Fisher Scientific, Waltham, USA) at 70-80% confluence. Then 24 h later, the cell monolayer was scratched with a yellow pipette tip. TGF-β1 (20 ng/ml) and AMD3100 (1 μg/ml) was added to each well. Images were analyzed at 0 and 24 h with a light microscope (Carl Zeiss Microimaging, Thornwood, NY, USA) and the scratch area was measured by the Image-Pro Plus software.
Measurement of ROS levels
CFBs were incubated with 10 μM 2’, 7’-dichlorofluorescein diacetate (DCF-DA, Sigma-Aldrich, USA) at 37°C for 30 min. The fluorescence intensities of DCF were monitored with a microscope (Carl Zeiss Microimaging, Thornwood, NY, USA) at excitation (488 nm) and emission (525 nm).
Statistical analysis
All data are presented as the mean ± SD of at least three experiments. Comparisons between two groups were analyzed with student's t-test (SPSS 23.0; SPSS Inc., Chicago, IL, USA). P<0.05 were considered to be statistically significant.
Results
The effect of AMD3100 on TGF-β1 induced the cell proliferation of CFBs
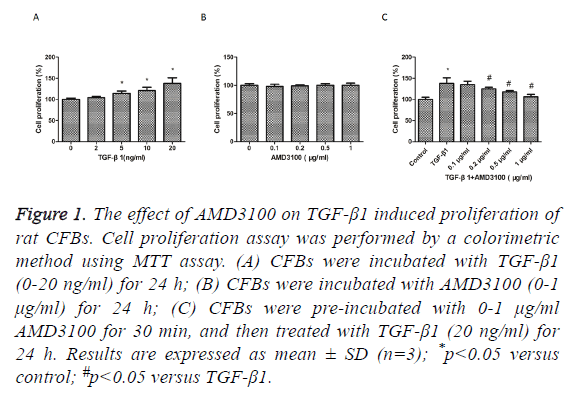
We first examined whether TGFβ1 and AMD3100 has cytotoxic effects in rat CFBs, the proliferation of CFBs were investigated by MTT assay. TGFβ1 treatment stimulated a substantially increase in CFBs proliferation compared with control group (Figure 1A). AMD3100 treatment did not affect CFBs proliferation (Figure 1B). However, TGFβ1induced increase in CFBs proliferation was suppressed by pre-treatment with AMD3100 (Figure 1C). So, AMD3100 (1 μg/ml) and TGF-β1 (20 ng/ml) were used as the optimal concentration for the next experiments.
Figure 1: The effect of AMD3100 on TGF-β1 induced proliferation of rat CFBs. Cell proliferation assay was performed by a colorimetric method using MTT assay. (A) CFBs were incubated with TGF-β1 (0-20 ng/ml) for 24 h; (B) CFBs were incubated with AMD3100 (0-1 μg/ml) for 24 h; (C) CFBs were pre-incubated with 0-1 μg/ml AMD3100 for 30 min, and then treated with TGF-β1 (20 ng/ml) for 24 h. Results are expressed as mean ± SD (n=3); *p<0.05 versus control; #p<0.05 versus TGF-β1.
The effect of AMD3100 on TGF-β1 induced the cell migration of CFBs
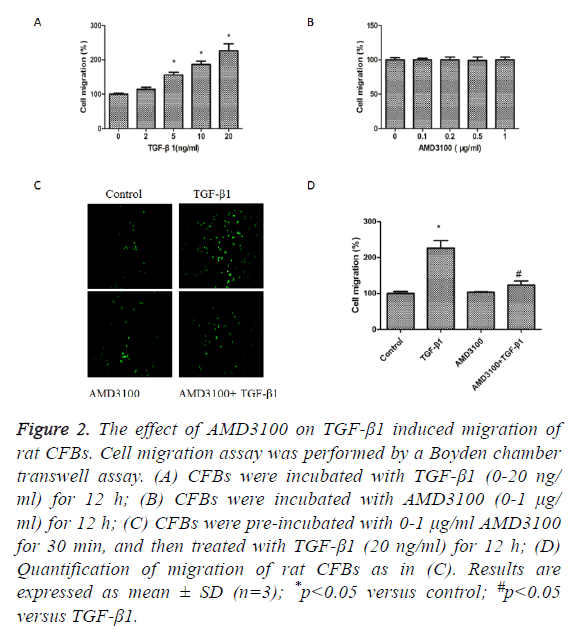
We next detected the effects of TGFβ1 and AMD3100 on migration of rat CFBs by a Boyden chamber transwell assay. TGFβ1 significantly induced CFBs migration (Figure 2A).
AMD3100 treatment did not affect CFBs migration (Figure 2B). However, TGFβ1induced increase in CFBs migration was inhibited by pre-treatment with AMD3100 (Figures 2C and 2D).
Figure 2: The effect of AMD3100 on TGF-β1 induced migration of rat CFBs. Cell migration assay was performed by a Boyden chamber transwell assay. (A) CFBs were incubated with TGF-β1 (0-20 ng/ ml) for 12 h; (B) CFBs were incubated with AMD3100 (0-1 μg/ ml) for 12 h; (C) CFBs were pre-incubated with 0-1 μg/ml AMD3100 for 30 min, and then treated with TGF-β1 (20 ng/ml) for 12 h; (D) Quantification of migration of rat CFBs as in (C). Results are expressed as mean ± SD (n=3); *p<0.05 versus control; #p<0.05 versus TGF-β1.
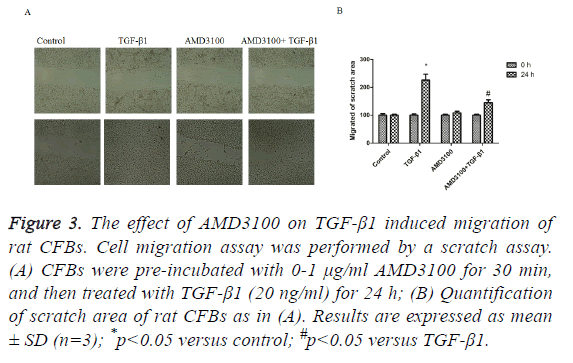
Next, we assessed CFBs migration by scratch assay. In the control group and AMD3100, there were no differences in migration of the CFBs (Figure 3). In the TGF-β1 group, CFBs showed significantly enhanced migration ability (Figure 3). However, TGFβ1 mediated increase in CFB migration was abolished by pre-treatment with AMD3100 (Figure 3), as presented by a wider scratch area.
Figure 3: The effect of AMD3100 on TGF-β1 induced migration of rat CFBs. Cell migration assay was performed by a scratch assay. (A) CFBs were pre-incubated with 0-1 μg/ml AMD3100 for 30 min, and then treated with TGF-β1 (20 ng/ml) for 24 h; (B) Quantification of scratch area of rat CFBs as in (A). Results are expressed as mean ± SD (n=3); *p<0.05 versus control; #p<0.05 versus TGF-β1.
The effect of AMD3100 on TGF-β1 induced the ROS generation of CFBs
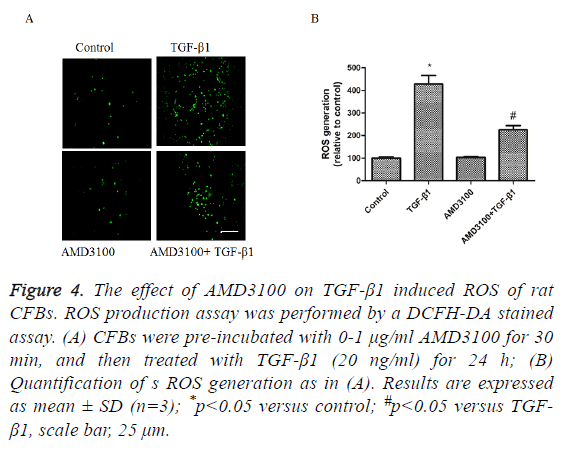
To detect the effects of TGFβ1 and AMD3100 on ROS generation of rat CFBs by a DCFH-DA stained assay. TGFβ1 significantly induced CFBs ROS generation (Figures 4A and 4B). AMD3100 treatment did not affect CFBs ROS generation (Figures 4A and 4B). However, TGFβ1 induced increase in CFBs ROS generation was inhibited by pre-treatment with AMD3100 (Figures 4A and 4B).
Figure 4: The effect of AMD3100 on TGF-β1 induced ROS of rat CFBs. ROS production assay was performed by a DCFH-DA stained assay. (A) CFBs were pre-incubated with 0-1 μg/ml AMD3100 for 30 min, and then treated with TGF-β1 (20 ng/ml) for 24 h; (B) Quantification of s ROS generation as in (A). Results are expressed as mean ± SD (n=3); *p<0.05 versus control; #p<0.05 versus TGF- β1, scale bar, 25 μm.
Discussion
This study demonstrated that AMD3100, CXCR4 antagonist, inhibits TGFβ1 stimulates proliferation and migration of rat CFBs. We also confirmed the molecular mechanisms as the activation of Akt pathway and reduction of ROS generation.
Activation of fibroblasts through proliferation and migration is important in wound healing and maturation of scar tissue after myocardial infarction [24,25]. Thus, the decreased CFBs proliferation and migration in the infarcted area might cause the deactivation of cardiac fibroblasts, which inhibits excessive fibrosis or delays the wound healing and maturation of scar tissue after myocardial infarction. TGFβ1 is an important signaling molecule that induces cardiac fibrosis by activating the proliferation and collagen production of CFBs [26,27]. The excessive TGFβ1 involved in the pathogenesis of maladaptive remodelling and fibrosis [28]. TGFβ1 binds to receptors and induces a number of signal pathways, including Akt signaling pathway. TGFβ1 also induce ROS generation, which activate the Akt signaling pathways [29]. So Akt signaling pathway has an important role in pathogenesis of CFBs.
In previous studies, the results demonstrated selective antagonism of the SDF-1/CXCR4 pathway reversed the development of cardiac fibrosis and left ventricular hypertrophy in the mice diabetes model [30]. In this study, AMD3100 had no effects on proliferation and migration in rat CFBs. It is thus suggested that AMD3100 has no cytotoxicity on rat CFBs. However, AMD3100 significantly inhibited TGFβ1 stimulates proliferation and migration in rat CFBs.
In conclusion, we revealed for the first time that AMD3100 attenuates TGFβ1 stimulates proliferation and migration of rat CFBs, at least partly via the activation of ROS pathway. These findings implied that CXCR4 might trigger cardiac fibroblast abnormal alteration and induce the process of cardiac fibrosis.
Acknowledgment
This study was supported by grants of the Shanghai Municipal Science and Technology Commission (No. 15ZR1412000) and Shanghai Municipal Commission of Health and Family Planning (20134371).
Conflict of Interest
The authors did not report any conflict of interest.
References
- Yuan J, Chen H, Ge D, Xu Y, Xu H, Yang Y. Mir-21 promotes cardiac fibrosis after myocardial infarction via targeting smad7. Cell Physiol Biochem 2017; 42: 2207-2219.
- Zhang F, Dang Y, Li Y, Hao Q, Li R, Qi X. Cardiac contractility modulation attenuate myocardial fibrosis by inhibiting TGF-beta1/Smad3 signaling pathway in a rabbit model of chronic heart failure. Cell Physiol Biochem 2016; 39: 294-302.
- Zhang H, Hui H, Li Z, Pan J, Jiang X, Wei T. Pigment epithelium-derived factor attenuates myocardial fibrosis via inhibiting endothelial-to-mesenchymal transition in rats with acute myocardial infarction. Sci Rep 2017; 7: 41932.
- Zhang JS, Hou YL, Lu WW, Ni XQ, Lin F, Yu YR. Intermedin1-53 protects against myocardial fibrosis by inhibiting endoplasmic reticulum stress and inflammation induced by homocysteine in apolipoprotein E-deficient mice. J Atheroscler Thromb 2016; 23: 1294-1306.
- Liu X, Liang E, Song X, Du Z, Zhang Y, Zhao Y. Inhibition of Pin1 alleviates myocardial fibrosis and dysfunction in STZ-induced diabetic mice. Biochem Biophys Res Commun 2016; 479: 109-115.
- Li X, Han D, Tian Z, Gao B, Fan M, Li C. Activation of cannabinoid receptor type II by AM1241 ameliorates myocardial fibrosis via Nrf2-mediated inhibition of TGF-beta1/Smad3 pathway in myocardial infarction mice. Cell Physiol Biochem 2016; 39: 1521-1536.
- Luo S, Hieu TB, Ma F, Yu Y, Cao Z, Wang M. ZYZ-168 alleviates cardiac fibrosis after myocardial infarction through inhibition of ERK1/2-dependent ROCK1 activation. Sci Rep 2017; 7: 43242.
- Zhang J, Li Z, Chen F, Liu H, Wang H, Li X. TGF-beta1 suppresses CCL3/4 expression through the ERK signaling pathway and inhibits intervertebral disc degeneration and inflammation-related pain in a rat model. Exp Mol Med 2017; 49: e379.
- Redondo S, Santos-Gallego CG, Tejerina T. TGF-beta1: a novel target for cardiovascular pharmacology. Cytokine Growth Factor Rev 2007; 18: 279-286.
- Guo Y, Dong Z, Shi Y, Wang W, Wang L, Sun J. Sonodynamic therapy inhibits fibrogenesis in rat cardiac fibroblasts induced by TGF-beta1. Cell Physiol Biochem 2016; 40: 579-588.
- Jiao H, Dong P, Yan L, Yang Z, Lv X, Li Q. TGF-beta1 induces polypyrimidine tract-binding protein to alter fibroblasts proliferation and fibronectin deposition in keloid. Sci Rep 2016; 6: 38033.
- Li J, Zhang W, Jiao R, Yang Z, Yuan Y, Wu Q. DIM attenuates TGF-beta1-induced myofibroblast differentiation in neonatal rat cardiac fibroblasts. Int J Clin Exp Pathol 2015; 8: 5121-5128.
- Johnston EF, Gillis TE. Transforming growth factor beta-1 (TGF-beta1) stimulates collagen synthesis in cultured rainbow trout cardiac fibroblasts. J Exp Biol 2017; 220: 2645-2653.
- Dutta A, Li J, Fedele C, Sayeed A, Singh A, Violette SM. Alphavbeta6 integrin is required for TGFbeta1-mediated matrix metalloproteinase2 expression. Biochem J 2015; 466: 525-536.
- Zhao X, Wang K, Liao Y, Zeng Q, Li Y, Hu F. MicroRNA-101a inhibits cardiac fibrosis induced by hypoxia via targeting TGF beta RI on cardiac fibroblasts. Cell Physiol Biochem 2015; 35: 213-226.
- Chung CC, Kao YH, Liou JP, Chen YJ. Curcumin suppress cardiac fibroblasts activities by regulating proliferation, migration, and the extracellular matrix. Acta Cardiol Sin 2014; 30: 474-482.
- Witte D, Bartscht T, Kaufmann R, Pries R, Settmacher U, Lehnert H. TGF-beta1-induced cell migration in pancreatic carcinoma cells is RAC1 and NOX4-dependent and requires RAC1 and NOX4-dependent activation of p38 MAPK. Oncol Rep 2017.
- Wang XW, Yu Y, Gu L. Dehydroabietic acid reverses TNF-alpha-induced the activation of FOXO1 and suppression of TGF-beta1/Smad signaling in human adult dermal fibroblasts. Int J Clin Exp Pathol 2014; 7: 8616-8626.
- Gupta SS, Zeglinski MR, Rattan SG, Landry NM, Ghavami S, Wigle JT. Inhibition of autophagy inhibits the conversion of cardiac fibroblasts to cardiac myofibroblasts. Oncotarget 2016; 7: 78516-78531.
- Di Maggio S, Milano G, De Marchis F, D'Ambrosio A, Bertolotti M, Palacios BS. Non-oxidizable HMGB1 induces cardiac fibroblasts migration via CXCR4 in a CXCL12-independent manner and worsens tissue remodeling after myocardial infarction. Biochimica et biophysica acta 2017; 1863: 2693-2704.
- Nishimura Y, Ii M, Qin G, Hamada H, Asai J, Takenaka H. CXCR4 antagonist AMD3100 accelerates impaired wound healing in diabetic mice. J Invest Dermatol 2012; 132: 711-720.
- Feng YF, Yuan F, Guo H, Wu WZ. TGF-beta1 enhances SDF-1-induced migration and tube formation of choroid-retinal endothelial cells by up-regulating CXCR4 and CXCR7 expression. Mol Cell Biochem 2014; 397: 131-138.
- Chu CY, Sheen YS, Cha ST, Hu YF, Tan CT, Chiu HC. Induction of chemokine receptor CXCR4 expression by transforming growth factor-beta1 in human basal cell carcinoma cells. J Dermatol Sci 2013; 72: 123-133.
- Okada M, Oba Y, Yamawaki H. Endostatin stimulates proliferation and migration of adult rat cardiac fibroblasts through PI3K/Akt pathway. Eur J Pharmacol 2015; 750: 20-26.
- Zhao J, Lei H. Tripartite motif protein 72 regulates the proliferation and migration of rat cardiac fibroblasts via the transforming growth factor-beta signaling pathway. Cardiology 2016; 134: 340-346.
- Stawowy P, Margeta C, Kallisch H, Seidah NG, Chretien M, Fleck E. Regulation of matrix metalloproteinase MT1-MMP/MMP-2 in cardiac fibroblasts by TGF-beta1 involves furin-convertase. Cardiovasc Res 2004; 63: 87-97.
- Yu M, Zheng Y, Sun HX, Yu DJ. Inhibitory effects of enalaprilat on rat cardiac fibroblast proliferation via ROS/P38MAPK/TGF-beta1 signaling pathway. Molecules 2012; 17: 2738-2751.
- Mewhort HE, Lipon BD, Svystonyuk DA, Teng G, Guzzardi DG, Silva C. Monocytes increase human cardiac myofibroblast-mediated extracellular matrix remodeling through TGF-beta1. Am J Physiol Heart Circ Physiol 2016; 310: 716-724.
- Liu YN, Zha WJ, Ma Y, Chen FF, Zhu W, Ge A. Galangin attenuates airway remodelling by inhibiting TGF-beta1-mediated ROS generation and MAPK/Akt phosphorylation in asthma. Sci Rep 2015; 5: 11758.
- Chu PY, Walder K, Horlock D, Williams D, Nelson E, Byrne M. CXCR4 antagonism attenuates the development of diabetic cardiac fibrosis. PloS One 2015; 10: e0133616.