Review Article - Current Trends in Cardiology (2017) Volume 1, Issue 2
Current understanding of the electrocardiographic manifestations of the ?athlete?s heart?.
Gemma Parry-Williams and Sanjay Sharma*
Cardiology Clinical and Academic Group, St. George’s University of London, Cranmer Terrace, London, UK
- *Corresponding Author:
- Sanjay Sharma
Cardiology Clinical and Academic group, St. George’s
University of London
Cranmer Terrace, London, UK
E-mail: sasharma@sgul.ac.uk
Accepted on September 18, 2017
DOI: 10.35841/cardiology.1.2.60-68
Visit for more related articles at Current Trends in CardiologyAbstract
This review will describe those ECG patterns within the normal spectrum for an athlete, describe the impact of demographic phenotypes on ECG interpretation and define those ECG manifestations that are always considered abnormal and warrant further investigation.
Keywords
Athlete’s heart, Electrocardiogram, International recommendations, Sudden cardiac death.
Introduction
Individuals who engage in regular, moderate to intensive exercise for between 4-8 hours per week can develop a constellation of physiological adaptations in cardiac structure, function and autonomic tone, collectively referred to as ‘The Athlete’s Heart’ [1]. Recognised ECG manifestations in athletes are attributable to increased vagal tone and increased chamber size and include sinus bradycardia, sinus arrhythmia, voltage criteria for ventricular enlargement, incomplete right bundle branch block and the early repolarisation pattern [2]. The electrocardiographic manifestations in athletes vary according to the type of sport and training intensity and also with the demographics of the athlete including; age, sex and ethnicity [3,4]. Occasionally, the ECG changes in athletes overlap with those considered characteristic of cardiomyopathies and ion channelopathies, which are recognised causes of exercise-related sudden cardiac death (SCD) in young athletes. Although sudden cardiac death in sport affects approximately 1 in 50,000 athletes, [5] the visibility afforded by these tragedies has led the European society of cardiology [6] and several sporting organisations around the world to advocate cardiovascular screening to identify athletes who may be at risk of sudden death. The ECG is considered an important tool for detecting high risk athletes, therefore, differentiation of those ECG phenotypes that reflect cardiac adaptation to exercise from those that indicate quiescent cardiac pathology is crucial. Athletes with an abnormal ECG are subject to comprehensive evaluation including cardiac imaging, exercise testing, prolonged rhythm monitoring, familial evaluation, genetic testing and reassessment after a period of detraining, may be necessary to differentiate between cardiac physiology and pathology. The stakes of confirming or refuting the diagnosis of a potentially serious cardiac disease in an athlete are high. An incorrect diagnosis would not only cause unnecessary stress to the athlete but could result in erroneous disqualification and termination of a career. Of even greater concern, is the false reassurance of an athlete harbouring disease implicated in young sudden cardiac death. It is important to provide clear guidance of the interpretation of the athlete’s ECG to physicians involved in evaluating young athletes.
Training Related ECG Changes in Trained Athletes
The physiological adaptations namely high vagal tone, increased chamber size and wall thickness, that occur as a consequence of chronic regular training of 4-8 h per week, are manifest on the athlete’s ECG [7]. In contrast, these changes in a sedentary individual, would be deemed abnormal and necessitate investigation to exclude cardiac pathology.
Vagotonia
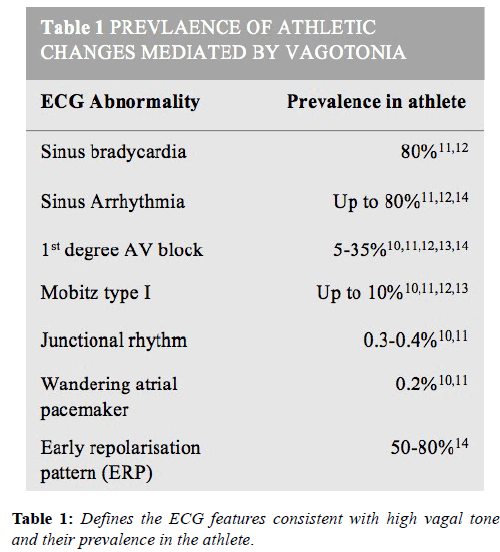
Conditioned athletes develop higher vagal tone which results in a greater prevalence of benign conduction anomalies. These include; sinus bradycardia (as slow as 30 bpm), sinus arrhythmia, ectopic atrial rhythm, junctional rhythms, first degree atrioventricular (AV) block (PR interval<400 ms) and Mobitz type 1 second degree AV block [8]. A finding in athletes not to be confused with complete heart block is AV mismatch, where as a consequence of high vagal tone, the AV node discharges and captures the ventricle faster than the sinoatrial node, with consequent AV dissociation. These changes are most prevalent in endurance athletes and resolve with both detraining and during sympathetic stress such as exercise. Unless accompanied by symptoms, they do not require any further work-up. Higher degrees of AV block such and Mobitz II and third degree heart block are extremely rare and always warrant further investigation [9-14].
Voltage criteria for left ventricular hypertrophy (LVH)
An increased QRS voltage is detected in up to 50% of athletes, most often in young, slender males. The presence of Sokolow- Lyon voltage criteria for LVH i.e. sum of S wave in V1 and R wave in V5 or V6>3.5 mV, rarely correlates with increased LV wall thickness [15]. Voltage criteria for LVH is also a recognised ECG manifestation of hypertrophic cardiomyopathy but, it is usually accompanied by ST-segment depression, inverted T-waves in the inferior-lateral leads and pathological Q-waves, which are not features of athletic training [16]. Therefore, isolated voltage criteria for LVH are considered normal in athlete’s but when accompanied by other pathological abnormalities warrants further investigation (Table 1).
Early repolarisation pattern (ERP)
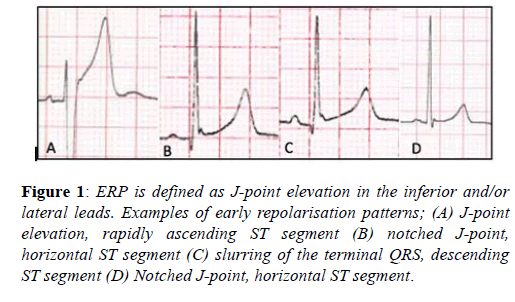
The early repolarisation pattern (ERP) is defined as J-point elevation, notching of the J-point or slurring of the terminal QRS, with or without ST-segment elevation, specifically in the inferior and/or lateral leads [17]. The ERP is recognised in up to 6% of the general population and between 23-44% of athletes, with a preponderance for young, black, male athletes and endurance athletes [9,18]. Until recently the ERP was considered a benign phenomenon [19], however three studies in the general population demonstrated a significantly higher prevalence of ERP’s, specifically in the inferior-lateral leads, amongst victims of idiopathic ventricular fibrillation. These studies failed however to demonstrate any association with increased recurrent events to support causality [20-23].
Overall these studies suggest a more malignant tendency of ERPs with horizontal or descending ST-segments and an inferior-lateral distribution. Amongst athletes the ERP is more prevalent at lower heart rates, is inducible with endurance training and has not been associated with adverse events [9]. Therefore, although further study is required, current evidence would suggest the ERP should be considered normal in athletes unless there is a history of unheralded syncope or a family history of a premature sudden death (Figure 1).
Figure 1: ERP is defined as J-point elevation in the inferior and/or lateral leads. Examples of early repolarisation patterns; (A) J-point elevation, rapidly ascending ST segment (B) notched J-point, horizontal ST segment (C) slurring of the terminal QRS, descending ST segment (D) Notched J-point, horizontal ST segment.
Incomplete RBBB
Incomplete right bundle branch block (RBBB) is reported in up to 60% of athletes. This finding is believed to be secondary to a mild conduction delay as a consequence of increased right ventricular (RV) cavity size, and in isolation does not warrant investigation [24,25].
Demographic Variations in ECG Phenotype
A number of factors including age, gender, ethnicity, training intensity and sporting discipline influence the ECG phenotype and should guide the interpretation of the athletes’ ECG.
The ‘juvenile’ athlete (age<16)
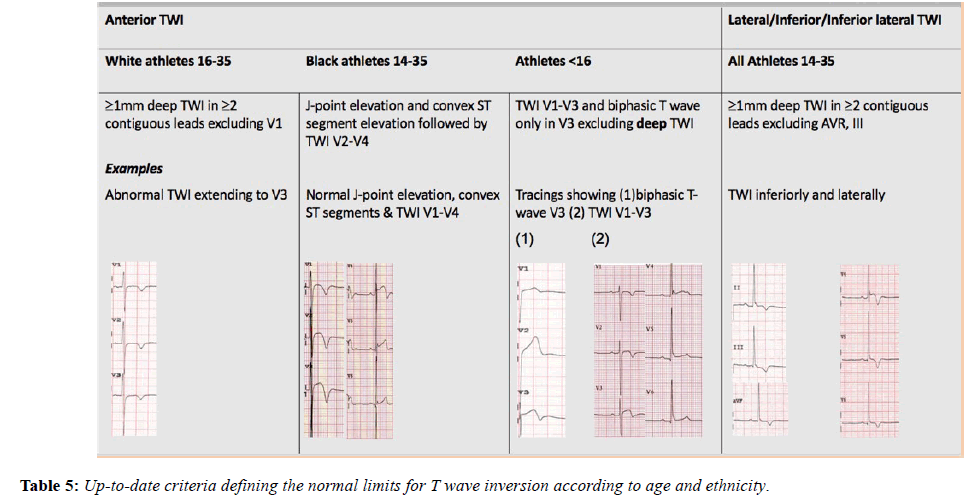
Anterior TWI extending beyond V2 is seen in between 4 and 9.5% of adolescent athletes and has not been associated with an increased risk of SCD. In the adult athlete, T wave inversion (TWI) beyond V2 it is much rarer (0.1%) and considered abnormal. TWI in V1 to V3 or an isolated biphasic T wave in V3 in an adolescent under 16 years old is considered normal and does not warrant further investigation unless T wave inversion beyond V2 persists after this age. In such cases comprehensive investigation is required to exclude arrythmogenic cardiomyopathy.
The black athlete
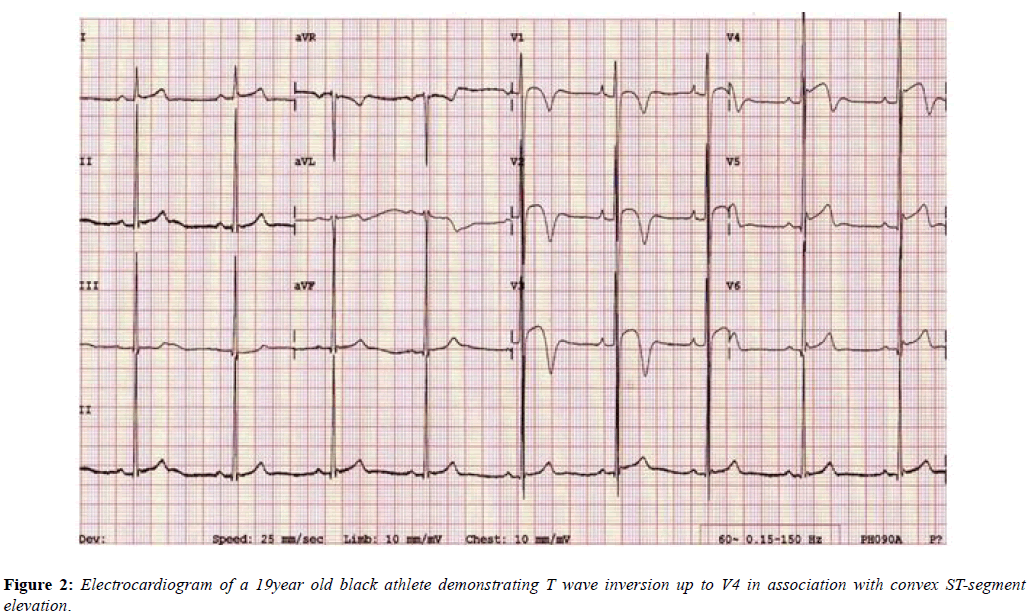
The Black athletes’ ECG more frequently shows electrical anomalies that may overlap with cardiac disease. Black athletes are 5 times more likely to have an abnormal ECG than White athletes [26] and are amongst those at greatest risk of exercise-related SCD. Their most common ECG pattern is anterior T wave inversion preceded by J-point elevation and convex ST-segments in V1-V4. In addition [27], Black athletes are more likely to meet criteria for LVH, right ventricular hypertrophy (RVH), right atrial enlargement, left atrial enlargement and early repolarisation compared with White athletes [28,29]. These changes are equally applicable to females as males (Figure 2) [30].
The female athlete
ECG changes including sinus bradycardia, voltage criteria for LVH and early repolarisation are less common in women, as is the accompanying degree of structural adaptation. Whether this is as a result of genetics, hormones or exercise ability is unclear. Conversely, female athletes show a higher prevalence of anterior TWI extending beyond V2 and a longer QT interval compared to male athletes [31-33].
The endurance athlete
Up to 35% of participants in endurance sports have an abnormal ECG. This is likely to reflect the profound physiological adaptation as a consequence of an intense and prolonged load on the heart [32]. Common findings include sinus bradycardia and the ERP. An important finding, due to its overlap with arrhythmogenic cardiomyopathy of the right ventricular form, is anterior TWI extending to V3, which is much more common when compared to strength athletes [33].
Abnormal ECG parameters in athletes
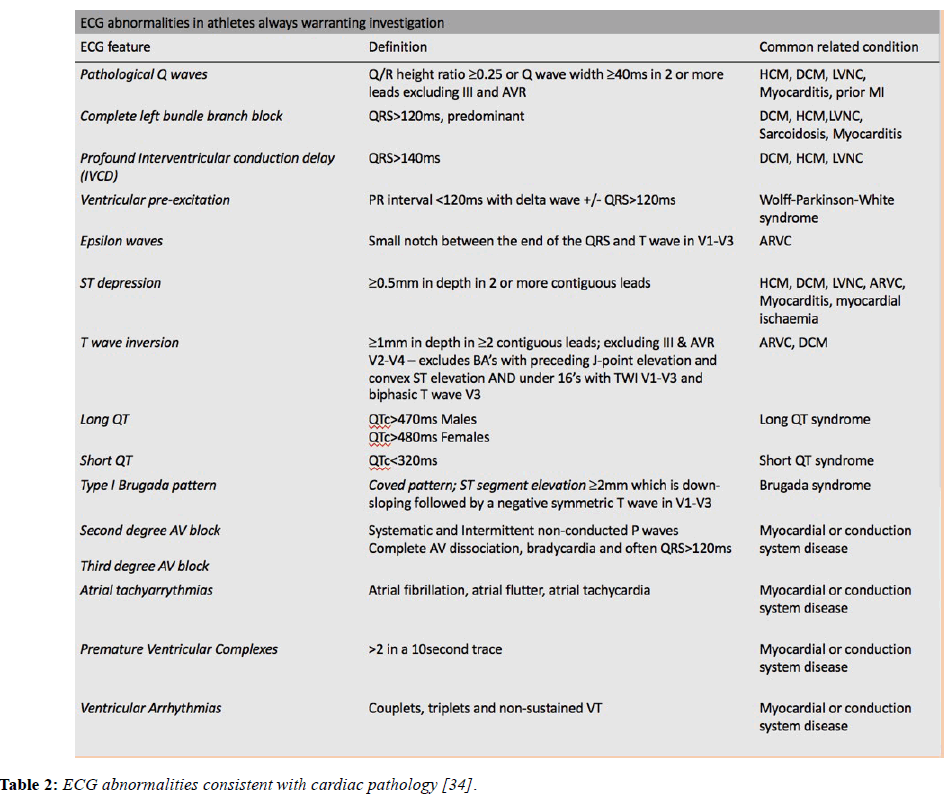
Although the ECG findings described above can be accounted for by athletic conditioning, there are a number of significant ECG changes that should not be attributed to training until an electrical or structural cardiac condition predisposing to sudden death has been excluded. The table below lists and defines the abnormal ECG features and the associated pathological diagnoses (Table 2).
Table 2: ECG abnormalities consistent with cardiac pathology [34].
T-wave inversion
T-wave inversion is one of the commonest ECG manifestations of cardiomyopathies including HCM and arrythmogenic cardiomyopathy, therefore TWI ≥ 1 mm in depth in 2 or more contiguous leads (excluding AVR, V1 and III) in the anterior, inferior or lateral territories always requires comprehensive evaluation. As already discussed anterior T-wave inversion of>1 mm depth, extending beyond V2 in a white athlete over the age of 16 is abnormal and warrants investigation. In the Black athlete however, TWI when preceded by J-point elevation and convex ST segments in considered normal up to V4 and equally TWI upto V3 in an adolescent athlete under 16 years old is also normal [28]. The presence of a depressed STsegment preceding TWI in the anterior leads however, is more suggestive of arrythmogenic cardiomyopathy of the right ventricular form and a helpful discriminator. Using other recognised ECG parameters of arrythmogenic cardiomyopathy including pathological Q waves, small amplitude complexes (<1.8 mV) in the precordial leads and ≥≥1 ventricular ectopic beat per 10 second tracing, will further assist in the differentiation of physiological adaptation from cardiac pathology [1,35].
Regardless of age, inferior-lateral TWI in the athlete of >1 mm in >2 contiguous leads (except III) is highly suspicious of pathology and always requires further evaluation. Left bundle block branch (LBBB) and ST depression. Left bundle branch block and ST depression should always be considered as abnormal and are frequently observed in individuals with cardiac pathology. They should always be investigated comprehensively to exclude both ischaemia and cardiomyopathies.
Pathological Q waves
Pathological Q waves are seen in the context of myocardial diseases such as HCM, myocardial infarction and Wolff- Parkinson-White syndrome. There has previously been a lack of consensus regarding the definition of pathological Q waves. The older Seattle criteria defined Q waves as >3 mm in depth or ≥≥ 40 ms in 2 or more contiguous leads, but when this was applied it resulted in a high-false positive rate due to deep Q waves occurring in the inferior-lateral leads in the context of LVH. The most recent international criteria define a pathological Q wave as one with a Q/R amplitude ratio ≥≥ 0.25 or Q wave width ≥≥ 40 ms in 2 or more leads. Such criteria should not apply to leads III and aVR or aVL if the QRS complexes are small [36].
Pre-excitation
Approximately one in two hundred and fifty athletes demonstrate pre-excitation on their ECG [37,38]. Due to the increased prevalence of AF in endurance athletes and the consequent risk of pre-excited AF and SCD due to VF, it is important to confidently exclude an accessory pathway that has the capacity to rapidly conduct in these individuals.
Therefore, the presence of pre-excitation on the ECG, regardless of symptoms, should be referred for further evaluation using exercise testing and electrophysiology studies, where necessary, to identify and thence treat those rapidly conducting pathways. Due to the rare association of preexcitation with cardiomyopathies and Epstein’s anomaly the clinical work-up should also include a transthoracic echocardiogram. Noteworthy is the fact that athletes commonly exhibit a PR interval of <120 ms; however, a short PR interval in isolation does not warrant any further action in an asymptomatic athlete [39].
Long QT interval
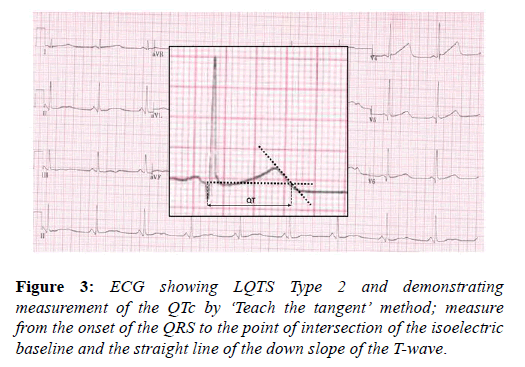
The long QT syndrome (LQTS) is an inherited ion channel disease affecting predominantly potassium channels and should be considered in athletes with a long QT interval, in whom drug and electrolyte causes have been excluded. Although difficult to demonstrate, LQTS is likely to be implicated in a significant proportion of cases of sudden cardiac death with a structurally normal heart. The high circulating levels of adrenaline present during exercise may induce ventricular arrhythmias, as can intense emotion and fear. Young athletes generally have a longer QT interval compared with the sedentary population and this may be secondary to increased left ventricular mass. The QTc should be corrected using the Bazett’s formula i.e. QTc=QT/√√RR. It is important to acknowledge that this method is inaccurate at heart rates <50 and >90 bpm and efforts should be made to perform the ECG at heart rates within these parameters. Other confounders to accurate determination of the QT interval common to athletes are; RR variability in sinus arrhythmia, with a need to average values, and prominent U waves, which should not be included in the measurement but can occasionally, is difficult to differentiate from the T wave. The QT interval should be systematically measured in lead II or V5 and physicians should adopt the ‘teach the tangent’ method for measurement, as illustrated in the image below. The cut off values for a long QT interval in athletes is set at the 99th percentile for the general population and is >470 ms in males and >480 ms in females (Figure 3).
A prolonged QT interval in an athlete warrants investigation including exercise testing, to assess for paradoxical QT prolongation during the early stages of exercise (up to a heart rate of 120 beats per minute) and notching of the T-wave, both of which are highly suggestive of long QT syndrome [40]. A QTc of ≥≥ 500 ms, without another explanation and regardless of symptoms is highly suggestive of LQTS [41]. A shorter QT interval i.e. 470-500 ms in males and 480-500 ms in females, in the context of syncope, a family history of LQTS or SADS is also diagnostic.
Type 1 Brugada pattern
Brugada syndrome is an inherited cardiac sodium ion channel disorder which predisposes to potentially fatal ventricular arrhythmias, particularly in states of high vagal tone and increased core body temperatures, both of which athletes are prone to. The characteristic ECG pattern in Brugada syndrome is J-point elevation 2 mm and a coved ST segment elevation that is down-sloping, followed by a negative symmetric T wave in leads V1-V3.
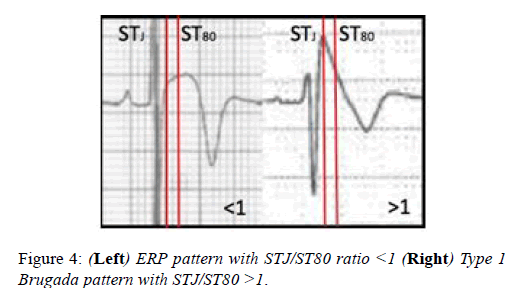
Athletes with the Brugada ECG pattern should be assessed for syncope and polymorphic ventricular tachycardia. The J-point elevation and convex ST segment elevation in leads V1-V3 seen in some Black athletes may raise the suspicion of the Brugada ECG pattern. In contrast with the Brugada ECG pattern, where there is a steep descent of the ST segment 80milliseconds after the J-point, the convex ST elevation in Black athletes continues to increase after the J-point (Figure 4). The ratio of the ST segment at the J-point (STj) and ST segment 80 milliseconds after the J-point is >1 in the Brugada syndrome and <1 in the Black athletes ECG (Figure 4).
The type 2 Brugada pattern has a saddleback appearance and is much more difficult to differentiate from athletic changes, particularly the repolarisation changes seen in black athlete’s. In such cases the ECG should be repeated with leads V1 and V2 placed in the second and third intercostal spaces to check for the characteristic type 1 Brugada pattern. Current recommendations advice no further testing in asymptomatic athletes with no family history and the type 2 pattern.
The Evolving ECG Interpretation Criteria in Athletes
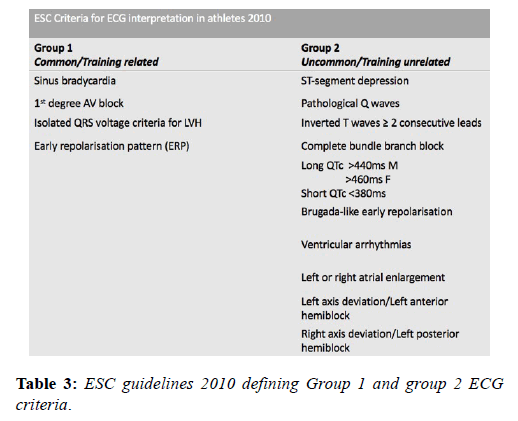
The prolific volume of research involving the ECG in the athlete’s heart in recent decades has resulted in frequent updates of the criteria for differentiating normal and abnormal and led to significant reductions in false positive rates and greater disease detection. A European Society of Cardiology (ESC) collaborative in 2010 [14] reported the first formal criteria for the interpretation of the ECG in athletes based upon the observations of an Italian study of 32,652 patients [37]. The consensus group subdivided ECG patterns into 2 groups based on their prevalence; ‘Group 1’ changes were those deemed physiological variants and did not require any further investigation and ‘Group 2’ changes were those rarely detected in athletes (5%), considered unrelated to training and therefore, warranting further evaluation (Table 3).
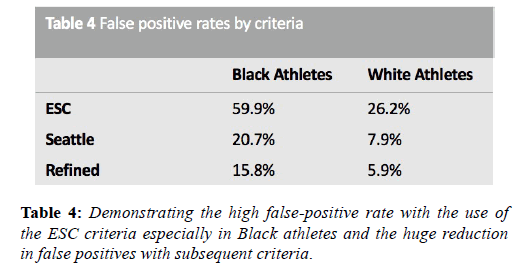
The application of these guidance resulted in unsatisfactorily high false positive rates, particularly among adolescent and Black athletes, because all T wave inversion was categorised as abnormal. Furthermore, the QT interval limits were identical to those for the general population. In 2012 the Seattle criteria made several amendments to the ESC 2010 recommendation criteria. These criteria classified TWI in leads V1-V4 with preceding dome-shaped ST elevation, as normal in Black athletes. In the absence of preceding dome-shaped ST elevation, anterior T wave inversion extending beyond V2 in Black athletes, as for White athletes, is also considered abnormal and necessitates further investigation. Other amendments included less conservative normal ranges for QTc (males 470 ms, females 480 ms) and an increase in the upper limit for the QRS from 120 ms to 140 ms in the context of nonspecific intraventricular conduction delay [42]. Subsequent evaluation of these criteria in a cohort of 1078 subjects in Australia, with an 86% prevalence of Caucasians, showed that the refinements had been successful in reducing false positives from 17% to 4.2% [43]. Several other studies have shown a more than 50% reduction in the number of positive ECG’s with the Seattle criteria compared to the ESC 2010 recommendations.
Refined criteria
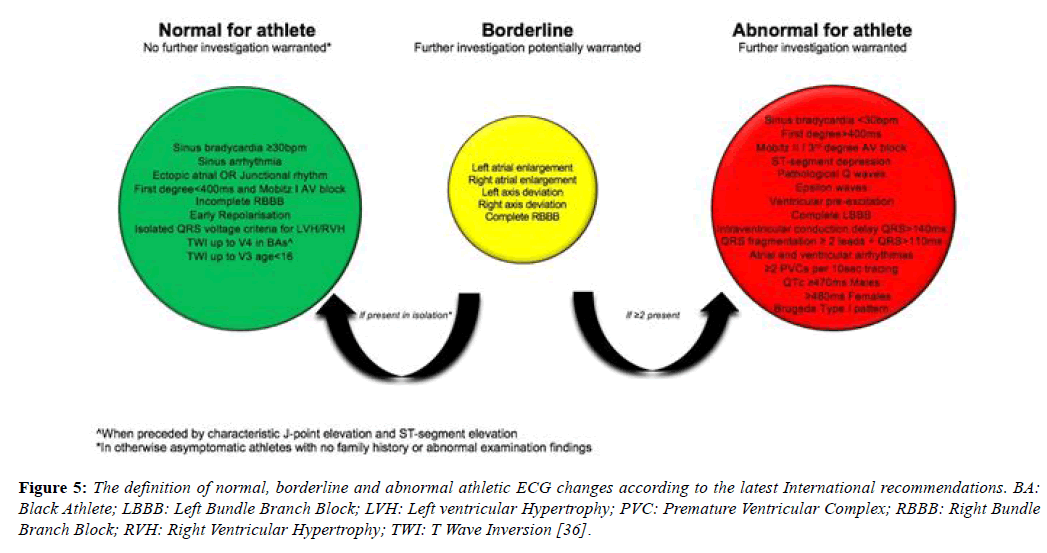
In 2014, the refined criteria introduced a third category containing ‘borderline’ ECG changes. These borderline changes included; (1) left atrial enlargement, (2) right atrial enlargement, (3) left axis deviation, (4) right axis deviation, (5) Sokolow-Lyon voltage criteria for right ventricular hypertrophy (RVH) and (6) TWI up to V4 in Black athletes (when preceded by convex ST segment elevation). It was stipulated that these criteria when found in isolation should be considered normal in asymptomatic athletes however, when co-existing with another borderline or group 2 criteria, further evaluation was indicated. Gati et al. provided scientific justification for these refinements, examining the ECG’s of 2533 young athletes and 10,000 non-athletes. There was a 13% prevalence of group 2 ESC criteria in athletes. Of these, 42.6% were due to isolated axis deviation or atrial enlargement and these were significantly more common in athletes. In the 598 subjects with abnormalities, echocardiography did not identify any significant cardiac abnormalities neither in athletes nor controls (Table 4). When isolated atrial enlargement and axis deviation were considered normal, false positives rates were reduced by 5.5% [44].
A further study of RVH in a cohort including athletes, patients with ARVC and pulmonary hypertension and controls, showed that no athlete with isolated RVH had any evidence of cardiac disease on echocardiography or cardiac MRI and additionally showed that none of those with ARVC and pulmonary hypertension had isolated RVH [45].
International recommendations
The international recommendations, published in February 2017, maintained the borderline category but have transferred anterior T wave inversion in Black athletes and voltage criteria into the normal category and right bundle branch block into the borderline category [46]. These recommendations also provide more extensive recommendations regarding the interpretation of TWI, a common finding in pathologies including; ARVC (55-85%), HCM (up to 85%) (Table 5), Left ventricular noncompaction (LVNC) and myocarditis and hence a crucial component of ECG interpretation (Figure 5) [28,47-49].
Conclusion
This athlete’s ECG is governed by several demographic factors including the age, sex, ethnicity and sporting discipline. T wave inversion is responsible for the commonest diagnostic challenge in differentiating athlete’s heart from cardiomyopathy and largely affects black athletes and endurance athletes. Several large studies in young athletes have facilitated a better understanding of the electrical manifestations of the athlete’s heart and led to contemporary interpretation criteria that have improved the specificity of ECG screening without compromising specificity. Ideally, the interpretation of the ECG in athletes should be performed by experts in sports cardiology to minimize the risk of false positive results. . Further studies are required to investigate the precise cause and significance of T wave inversion in black athletes but there should also be focus on other ethnic groups and studies in master athletes.
References
- Zaidi A, Sheikh N, Jongman JK, et al. Clinical Differentiation Between Physiological Remodeling and Arrhythmogenic Right Ventricular Cardiomyopathy in Athletes With Marked Electrocardiographic Repolarization Anomalies. J Am Coll Cardiol. 2015;65:2702–11.
- Prakash K, Sharma S. Interpretation of the Electrocardiogram in Athletes. Can J Cardiol. Elsevier; 2016;32:438–51.
- Sharma S. Athlete's heart--effect of age, sex, ethnicity and sporting discipline. Experimental Physiology. 2003;88:665–9.
- Keteepe-Arachi T, Sheikh N. The Athlete's Heart: Impact of Age, Sex, Ethnicity and Sporting Discipline. 2016.
- Harmon KG, Drezner JA, Wilson MG, et al. Incidence of sudden cardiac death in athletes: a state-of-the-art review. Heart. 3rd ed. BMJ Publishing Group Ltd and British Cardiovascular Society; 2014;100:1227–34.
- Mont L, Pelliccia A, Sharma S, et al. Pre- participation cardiovascular evaluation for athletic participants to prevent sudden death: Position paper from the EHRA and the EACPR, branches of the ESC. Endorsed by APHRS, HRS, and SOLAECE. Vol. 19, Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2017. pp. 139–63.
- Fagard R. Athlete’s heart. Heart. BMJ Publishing Group Ltd and British Cardiovascular Society. 2003;89:1455–61.
- Mont L. Arrhythmias and sport practice. Heart. BMJ Publishing Group Ltd and British Cardiovascular Society. 2010;96:398–405.
- Noseworthy PA, Weiner R, Kim J, et al. Early repolarization pattern in competitive athletes: clinical correlates and the effects of exercise training. Circ Arrhythm Electrophysiol. American Heart Association, Inc. 2011;4:432–40.
- Northcote RJ, Canning GP, Ballantyne D. Electrocardiographic findings in male veteran endurance athletes. Br Heart J. 1989;61:155–60.
- Sharma S, Whyte G, Elliott P, et al. Electrocardiographic changes in 1000 highly trained junior elite athletes. Br J Sports Med. BMJ Publishing Group Ltd and British Association of Sport and Exercise Medicine. 1999;33:319–24.
- Papadakis M, Basavarajaiah S, Rawlins J, et al. Prevalence and significance of T-wave inversions in predominantly Caucasian adolescent athletes. European Heart Journal. The Oxford University Press. 2009;30:1728–35.
- Meytes I, Kaplinsky E, Yahini JH, et al. Wenckebach A-V block: a frequent feature following heavy physical training. Am Heart J. Elsevier. 1975;90:426–30.
- Corrado D, Pelliccia A, Heidbuchel H, et al. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Vol. 31, European Heart Journal. The Oxford University Press; 2010.
- Sohaib SMA, Payne JR, Shukla R, et al. Electrocardiographic (ECG) criteria for determining left ventricular mass in young healthy men; data from the LARGE Heart study. J Cardiovasc Magn Reson. BioMed Central. 2009;11:2.
- Ryan MP, Cleland JG, French JA, et al. The standard electrocardiogram as a screening test for hypertrophic cardiomyopathy. Am J Cardiol. 1995;76:689–94.
- Macfarlane PW, Antzelevitch C, Haissaguerre M, et al. The Early Repolarization Pattern: A Consensus Paper. J Am Coll Cardiol. 2015;66:470–7.
- Adler A, Rosso R, Viskin D, et al. What do we know about the “malignant form” of early repolarization? J Am Coll Cardiol. 2013;62:863–8.
- Klatsky AL, Oehm R, Cooper RA, et al. The early repolarization normal variant electrocardiogram: correlates and consequences. The American Journal of Medicine. Elsevier; 2003;115:171–7.
- Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–23.
- Rosso R, Kogan E, Belhassen B, et al. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: incidence and clinical significance. J Am Coll Cardiol. 2008;52:1231–8.
- Tikkanen JT, Anttonen O, Junttila MJ, et al. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–37.
- Cappato R, Furlanello F, Giovinazzo V, et al. J wave, QRS slurring, and ST elevation in athletes with cardiac arrest in the absence of heart disease: marker of risk or innocent bystander? Circ Arrhythm Electrophysiol. American Heart Association, Inc. 2010;3:305–11.
- Wilson MG, Chatard JC, Carre F, et al. Prevalence of electrocardiographic abnormalities in West-Asian and African male athletes. Br J Sports Med. BMJ Publishing Group Ltd and British Association of Sport and Exercise Medicine. 2012;46:341–7.
- Langdeau JB, Blier L, Turcotte H, et al. Electrocardiographic findings in athletes: the prevalence of left ventricular hypertrophy and conduction defects. Can J Cardiol. 2001;17:655–9.
- Riding NR, Sheikh N, Adamuz C, et al. Comparison of three current sets of electrocardiographic interpretation criteria for use in screening athletes. Heart. BMJ Publishing Group Ltd and British Cardiovascular Society. 2015;101:384–90.
- Wilson MG, Sharma S, Carr F, et al. Significance of deep T-wave inversions in asymptomatic athletes with normal cardiovascular examinations: practical solutions for managing the diagnostic conundrum. Br J Sports Med. 2012;46:i51–8.
- Papadakis M, Carre F, Kervio G, et al. The prevalence, distribution, and clinical outcomes of electrocardiographic repolarization patterns in male athletes of African/Afro-Caribbean origin. European Heart Journal. The Oxford University Press. 2011;32:2304–13.
- DI PAOLO FM, Schmied C, Zerguini YA, et al. The athlete's heart in adolescent Africans: an electrocardiographic and echocardiographic study. J Am Coll Cardiol. 2012;59:1029–36.
- Rawlins J, Carre F, Kervio G, et al. Ethnic differences in physiological cardiac adaptation to intense physical exercise in highly trained female athletes. Circulation. American Heart Association, Inc. 2010;121:1078–85.
- Brosnan M, La Gerche A, Kalman J, et al. Comparison of Frequency of Significant Electrocardiographic Abnormalities in Endurance Versus Nonendurance Athletes. Am J Cardiol. Elsevier. 2014;113:1567–73.
- Pelliccia A, Maron BJ, Culasso F, et al. Clinical significance of abnormal electrocardiographic patterns in trained athletes. Circulation. American Heart Association, Inc. 2000;102:278–84.
- Malhotra A, Dhutia H, Gati S, Yeo TJ, et al. Anterior T-Wave Inversion in Young White Athletes and Nonathletes: Prevalence and Significance. J Am Coll Cardiol. 2017;69:1–9.
- Sharma S, Drezner JA, Baggish A, et al. International Recommendations for Electrocardiographic Interpretation in Athletes. J Am Coll Cardiol. 2017;69:1057–75.
- Calore C, Zorzi A, Sheikh N, et al. Electrocardiographic anterior T-wave inversion in athletes of different ethnicities: differential diagnosis between athlete's heart and cardiomyopathy. Eur Heart J. 2016;37:2515–27.
- Sharma S, Drezner JA, Baggish A, et al. International Recommendations for Electrocardiographic Interpretation in Athletes. J Am Coll Cardiol. 2017;69:1057–75.
- Pelliccia A, Culasso F, DI PAOLO FM, et al. Prevalence of abnormal electrocardiograms in a large, unselected population undergoing pre-participation cardiovascular screening. Eur Heart J. 2007;28:2006–10.
- Harmon KG, Asif IM, Klossner D, et al. Incidence of sudden cardiac death in National Collegiate Athletic Association athletes. Circulation. American Heart Association, Inc. 2011;123:1594–600.
- Drezner JA, Ackerman MJ, Cannon BC, et al. Abnormal electrocardiographic findings in athletes: recognising changes suggestive of primary electrical disease. Br J Sports Med. BMJ Publishing Group Ltd and British Association of Sport and Exercise Medicine. 2013;47:153–67.
- Lehmann MH, Suzuki F, Fromm BS, et al. T wave “humps” as a potential electrocardiographic marker of the long QT syndrome. J Am Coll Cardiol. 1994;24:746–54.
- Basavarajaiah S, Wilson M, Whyte G, et al. Prevalence and significance of an isolated long QT interval in elite athletes. Eur Heart J. 2007;28:2944–9.
- Drezner JA, Ackerman MJ, Anderson J, et al. Electrocardiographic interpretation in athletes: the 'Seattle criteria'. BMJ Publishing Group Ltd and British Association of Sport and Exercise Medicine; 2013.
- Brosnan M, La Gerche A, Kalman J, et al. The Seattle Criteria increase the specificity of preparticipation ECG screening among elite athletes. Br J Sports Med. BMJ Publishing Group Ltd and British Association of Sport and Exercise Medicine; 2014;48:1144–50.
- Gati S, Sheikh N, Ghani S, et al. Should axis deviation or atrial enlargement be categorised as abnormal in young athletes? The athlete's electrocardiogram: time for re-appraisal of markers of pathology. Eur Heart J. The Oxford University Press. 2013;34:3641– 8.
- Zaidi A, Ghani S, Sharma R, et al. Physiological right ventricular adaptation in elite athletes of African and Afro- Caribbean origin. Circulation. American Heart Association, Inc. 2013;127:1783–92.
- Sheikh N, Papadakis M, Ghani S, et al. Comparison of electrocardiographic criteria for the detection of cardiac abnormalities in elite black and white athletes. Circulation. American Heart Association, Inc. 2014;129:1637–49.
- Maron BJ, Doerer JJ, Haas TS, et al. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation. American Heart Association, Inc. 2009;119:1085–92.
- Östman-Smith I, Wettrell G, Keeton B, et al. Age- and gender-specific mortality rates in childhood hypertrophic cardiomyopathy. Eur Heart J. 2008;29:1160–7.
- Jain R, Dalal D, Daly A, et al. Electrocardiographic features of arrhythmogenic right ventricular dysplasia. Circulation. American Heart Association, Inc. 2009;120:477–87.