Review Article - International Journal of Pure and Applied Zoology (2022) Volume 10, Issue 11
CURRENT SCENARIO OF MONKEY POX VIRUS IN GLOBALLY : A REVIEW
Selvaraj Selvamurugan*
Department of Clinical Development Service Agency, Translational Health Science and Technology Institute Building (THSTI), Faridabad-121001, India
- Corresponding Author:
- Selvaraj Selvamurugan
Department of Clinical Development Service Agency
Translational Health Science and Technology Institute Building (THSTI)
Faridabad-121001, India
E-mail: selva199420@yahoo.in
Received: 11-Oct-2022, Manuscript No. IJPAZ-22-76821; Editor assigned: 13-Oct-2022, PreQC No. IJPAZ-22-76821(PQ); Reviewed: 27-Oct-2022, QC No. IJPAZ-22-76821; Revised: 01-Nov-2022, Manuscript No. IJPAZ-22-76821(R); Published: 08-Nov-2022, DOI: 10.35841/2320-9585-10.11.152
Abstract
Monkeypox is an emerging viral zoonosis with symptoms similar to those observed in smallpox patients, although less severe. Since the global eradication of smallpox in 1980, monkeypox has emerged as the most important orthopoxvirus in humans. Recently, there is large outbreak of monkeypox virus in Central Africa and this remains as a growing public health threat. The last confirmed case of monkeypox was in 1978 at Nigeria. Monkeypox is now a major threat to global health security, requiring an urgent multidisciplinary approach including virologists, veterinarians, physicians, and public health experts to fast?track the development of diagnostic assays, vaccines, antivirals, and other control strategies. This aim of this manuscript is to provide information on the current state of knowledge about human monkeypox, with emphasis on epidemiologic characteristics, clinical features, diagnosis, treatment, and prevention.
Keywords
Monkey Pox, Prevalence, Treatment, Symptoms, Disease.
Introduction
Poxviruses belong to family Poxviridae, a large and diverse family of double-stranded DNA viruses that multiplies in the cytoplasm of infected cells the poxviruses are known to have brick-shaped or oval structures measuring 200–400 nm when viewed with electron microscopy [1-5]. The wide host range of poxvirus, as well as their successful evolution, is partly due to their manipulation and modulation of host immune responses. The poxviruses are also called ancient viruses because they have been found in insects, reptiles, birds, and mammals. It is believed that these viruses, before the divergence of invertebrates and vertebrates, form visible “pox” [6,7].
The family Poxviridae is subdivided (based on their animal hosts) into two subfamilies, namely Chordopoxvirinae and Entomopoxvirinae. On 3 November 2017, WHO hosted an informal consultation on monkeypox in Geneva which brought together Ministries of Health of affected West and Central African countries, AFRO and country office staff, global health partners and orthopoxvirus experts to discuss the current situation, state of knowledge, identify needs and address critical gaps and challenges in combatting monkeypox outbreaks.
Thus, the objective of this paper is to review the current state of knowledge concerning the infection biology, epidemiology, and evolution of MPXV in country wise. In addition, this review also chronicles and explores the knowledge gaps as they pertain to MPXV reservoir hosts and the ecological dynamics that modulate the maintenance of the virus in nature, as well as their spillover into human populations and subsequent humanto- human transmission.
Background
Monkeypox is a re-emerging zoonotic infectious disease which appears to becoming more entrenched and widespread in regions where it has not been detected for decades. The virus was first identified as a naturally-occurring agent of human disease in 1970 in the Democratic Republic of Congo (DRC, formerly Zaire), and was subsequently documented in other countries of West and Central Africa. With the eradication of smallpox and subsequent cessation of routine smallpox vaccination in 1980, human monkeypox was extensively studied in DRC and was found to be a zoonotic virus (unlike variola virus which causes smallpox) with the ability for limited transmission from human to humans.
During the last five decades the majority of human infections have been reported from DRC where it is a reportable disease and now more than a thousand cases are reported annually. Prior to 2000, reports of human monkeypox outside of DRC were sparse—with 21 cases reported from 7 countries in West and Central Africa – most reported in the 1970’s and 1980’s. Since 2016, confirmed human monkey pox cases have been reported from Central African Republic, DRC, Liberia, Nigeria, Sierra Leone, and Republic of Congo. Many of these countries have not reported cases previously for several decades. Nigeria is currently facing the largest documented outbreak of West African monkey pox with 197 suspected cases and 68 confirmed cases. In addition, infections in wild and/or captive animals have also been detected in Cameroon, Cote d’Ivoire, and DRC, suggesting the risk of zoonotic transmission to humans from locally circulating virus.
Monkey pox virus was first reported in 1959 as an outbreak of a pox-like disease in monkeys kept at a research institute in Copenhagen, Denmark. The first human MPXV case in medical history was recognized when, on 1 September 1970, a ninemonth- old child was admitted to the Basankusu Hospital in the Democratic republic of Congo (at that time, known as the Republic of the Congo). The boy had a smallpox-like disease from which MPXV-like virus was isolated [8-11]. Six cases of human MPXV were described in Liberia, Nigeria, and Sierra Leone between October 1970 and May 1971. The first index MPXV case in Nigeria was recorded in 1971, and 10 MPXV cases were reported between 1971 and 1978 [12]. Since then, several thousand human cases of monkey pox have been confirmed in 15 different countries, with 11 of them in African countries. Monkey pox was imported to the United Kingdom, the USA, Israel, and Singapore [WHO 2020].
Mode of virus transmission
The monkey pox virus is transmitted to humans through a bite or direct contact with an infected animal’s blood, body fluids or cutaneous/mucosal lesions. Monkey pox virus transmission occurs when a person comes into contact with the virus from human, animal or materials contaminated with the virus. It was postulated that the virus enters the body through broken skin, mucous membrane or respiratory tract. Animal to human transmission is possible by bite or scratch, bush meat preparation or direct contact with body fluids or lesion material. Indirect contact with lesion material such as through contaminated bedding may also cause the virus transmission [13]. Human to human virus transmission is thought to occur mainly through large respiratory droplets. Respiratory droplets generally cannot travel more than a few feet, hence prolonged face toface contact is required for the virus transmission. Other human to human methods of transmission include direct or indirect contact with lesion material or body fluids.
The two possible means of MPXV transmission are animals– human transmission and human–human transmission. Respiratory droplets and contact with body fluids, contaminated patient’s environment or items, skin lesion of an infected person have been found to be associated with inter-human transmission. Congo Basin clade (Central Africa clade) is reported to be more virulent than West Africa clade and thereby contributes more to inter-human transmission [14-18].
Animal-to-human transmission, which is also known as zoonotic transmission, occurs via direct contact with any of the aforementioned natural viral hosts or consumption of these hosts. In addition, zoonotic transmission could occur by direct contact with the blood, body fluids, and inoculation from mucocutaneous lesions of an infected animal [19-22]. Nosocomial transmission has been reported for CB and WA clades of MPXV [23-25]. While sexual transmission has been speculated for infected individuals with groin and genital lesions [26]. At present human-to-animal transmission has not been reported. Human-to-human transmission, secondary attack rates (SARS), and serial transmission events are much higher with the CB clade compared to the WA clade [26].
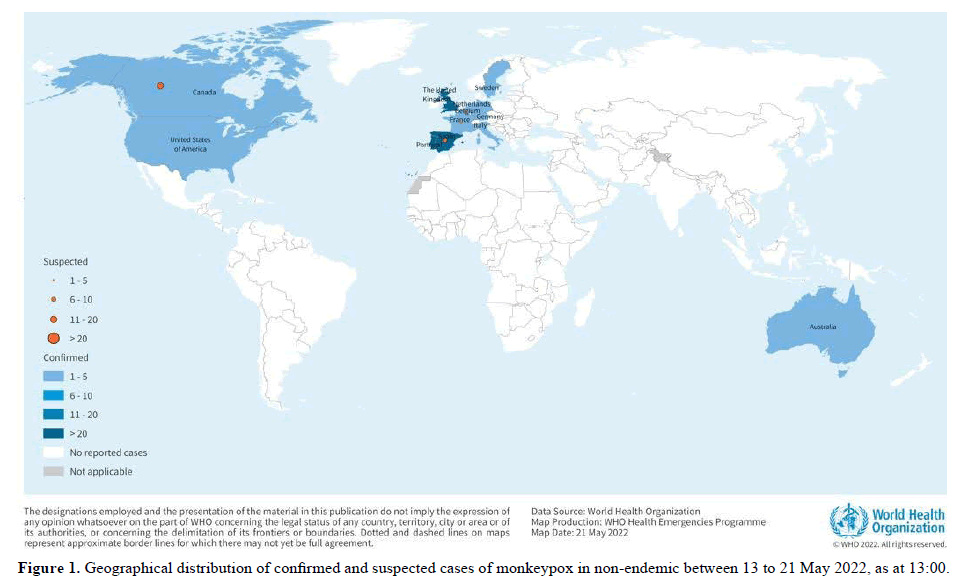
As of 21 May, 13:00, 92 laboratory confirmed cases, and 28 suspected cases of monkeypox with investigations ongoing, have been reported to WHO from 12 Member States that are not endemic for monkey pox virus, across three WHO regions (Table 1, Figure 1). No associated deaths have been reported to date.
| Country | Confirmed | Suspected |
|---|---|---|
| Australia | 1-5 | - |
| Belgium | 1-5 | 1-5 |
| Canada | 1-5 | 11-20 |
| France | 1-5 | 1-5 |
| Germany | 1-5 | - |
| Italy | 1-5 | - |
| Netherlands | 1-5 | - |
| Portugal | 21-30 | - |
| Spain | 21-30 | 6-10 |
| Sweden | 1-5 | - |
| United Kingdom | 21-30 | - |
| United States of America | 1-5 | - |
| Total | 92 | 28 |
Table 1. Cases of monkeypox in non-endemic countries reported to WHO between 13 to 21 May 2022 as at 13:00.
Animal reservoirs
Indirect or direct contact with live or dead animals is assumed to be the driver of human monkey pox infections in humans [27-29]. Monkey pox primarily occurs in animals in the equatorial rain forests in West Africa and Central Africa. In 1985, the virus was isolated from a moribund rope squirrel (Funisciurus anerythrus) in Zaire (DRC) during an outbreak investigation. Evidence of monkey pox infection has been found in a range of animal species: squirrels (rope and tree), rats, striped mice, dormice and monkeys. The specific animal host reservoir of monkey pox, the and surveillance capacities across Africa to guide appropriate surveillance, data collection, prevention, preparedness and response activities to monkey pox and other emerging and re-emerging infections with epidemic potential. Advancing public health preparedness and aligning proactive surveillance activities to priority research will require a coordinated, locally-led, multidisciplinary efforts aligned closely with capacity development and training (Table 2).
| Country | Time period | Cumulative cases | Cumulative deaths |
|---|---|---|---|
| Cameroon | 15 December 2021to 22 February 2022 |
25 | <5 |
| Central African Republic | 4 March to 10 April 2022 | 6 | <5 |
| Democratic Republic of the Congo |
1January to 1May 2022 | 1238 | 57 |
| Nigeria | 1January 2022 to 30 April 2022 | 46 | 0 |
Table 2. Cases of monkey pox in endemic countries between 15 December 2021 to 1 May 2022.
Diagnosis
The clinical differential diagnosis that must be considered includes other rash illnesses, such as chickenpox, measles, bacterial skin infections, scabies, syphilis, and medication-associated allergies. Lymphadenopathy during the prodromal stage of illness can be a clinical feature to distinguish monkey pox from chickenpox or smallpox.
If monkey pox is suspected, health workers should collect an appropriate sample and have it transported safely to a laboratory with appropriate capability. Confirmation of monkey pox depends on the type and quality of the specimen and the type of laboratory test. Thus, specimens should be packaged and shipped in accordance with national and international requirements. Polymerase chain reaction (PCR) is the preferred laboratory test given its accuracy and sensitivity. For this, optimal diagnostic samples for monkey pox are from skin lesions–the roof or fluid from vesicles and pustules, and dry crusts. Where feasible, biopsy is an option. Lesion samples must be stored in a dry, sterile tube (no viral transport media) and kept cold. PCR blood tests are usually inconclusive because of the short duration of viremia relative to the timing of specimen collection after symptoms begin and should not be routinely collected from patients.
Symptoms of illness
The incubation period is usually 6 to 21 days. The illness typically lasts for two to four weeks. It is characterised by fever, myalgia, headache, lymphadenopathy and rash. The rash which is first seen about two days after fever onset usually starts in the trunk and spreads peripherally to involve the palms and soles. The rash starts as a macules and papules and then progresses to become vesicles and pustules before scabbing and desquamation over a 2-3 week period. Unlike in chickenpox where lesions at various stages of development and healing are seen, in monkeypox all the lesions are generally at the same stage. Lymphadenopathy is observed prior to and concomitant with the rash, which helps differentiate it from smallpox or varicella [30].
One or more of the following signs or symptoms, since 15 March 2022:
• Headache
• Acute onset of fever (>38.5oC),
• Lymphadenopathy (swollen lymph nodes)
• Myalgia (muscle and body aches)
• Back pain
• Asthenia (profound weakness).
Therapeutics
Clinical care for monkeypox should be fully optimized to alleviate symptoms, manage complications and prevent long-term sequelae. Patients should be offered fluids and food to maintain adequate nutritional status. Secondary bacterial infections should be treated as indicated. An antiviral agent known as tecovirimat that was developed for smallpox was licensed by the European Medical Association (EMA) for monkeypox in 2022 based on data in animal and human studies. It is not yet widely available.
If used for patient care, tecovirimat should ideally be monitored in a clinical research context with prospective data collection.
Treatment for monkeypox infection
There is no specific treatment or vaccine for monkey pox infection. Monkey pox is usually a self-limited disease with the symptoms lasting from 2 to 3 weeks. Severe symptoms common among children and is related to extent of virus exposure and patient’s health status.
Conclusions
COVID-19 is the worst pandemic in scale and speed of this century associated with the highest number of global deaths, with most of the deaths reported in high income countries. Risk factors such as increasing age, obesity, and co morbidities including pulmonary diseases, diabetes, cancer and neurological diseases drive the infection fatality rate. Although the infection fatality rate is far lower compared to other emerging infectious dis- international travel was relentless despite early travel restrictions and travel bans. However, travel restrictions delayed the importation and reduced the outbreak size. Mobility restrictions continue to be used across countries. Due to the high reproduction number, combating COVID-19 will require an allsociety and all-government approach.
References
- Alakunle, E., Moens, U., Nchinda, G., and Okeke, M.I., 2020. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses., 12: 1257.
- Beer, E.M., and Rao, V.B., 2019. A systematic review of the epidemiology of human monkey pox outbreaks and implications for outbreak strategy. PLoS. Negl. Trop. Dis., 13: 1–20.
- Brown, K., and Leggat, P.A., 2016. Human monkeypox: current state of knowledge and implications for the future. Trop. Med. Infect. Dis., 1: 8.
- Hughes, A. L., Irausquin, S., and Friedman, R., 2010. The evolutionary biology of poxviruses. Infect. Genet. Evol., 10: 50–59.
- Diven, D.G., 2001. An overview of poxviruses. J. Am. Acad. Dermatol., 44: 1-16.
- Odom, M.R., Hendrickson, R.C., and Lefkowitz, E.J., 2009. Poxvirus protein evolution: family wide assessment of possible horizontal gene transfer events. Virus. Res., 144: 233-249.
- Essbauer, S., Pfeffer, M., and Meyer, H., 2010. Zoonotic poxviruses. Vet. Microbiol., 140: 229-236.
- Langohr, I.M., Stevenson, G.W., Thacker, H.L., and Regnery, R.L., 2004. Extensive lesions of monkeypox in a prairie dog (Cynomys sp.). Vet. Pathol., 41: 702-707.
- Breman, J.G., Steniowski, M.V., Zanotto, E., Gromyko, A.I., and Arita, I., 1980. Human monkeypox, 1970-79. Bull. World. Health. Organ., 58: 165.
- Jezek, Z., Gromyko, A.I., and Szczeniowski, M.V., 1983. Human monkeypox. J. Hyg. Epidemiol. Microbiol. Immunol., 27: 13-28.
- Ladnyj, I.D., Ziegler, P., and Kima, E., 1972. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World. Health. Organ. 46: 593–597.
- Foster, S.O., Brink, E.W., Hutchins, D.L., Pifer, J.M., Lourie, B., Moser, C.R., and Foege, W.H., 1972. Human monkeypox. Bull. World. Health. Organ., 46: 569.
- Khodakevich, L., Szczeniowski, M., Jezek, Z., Marennikova, S., Nakano, J., and Messinger, D., 1987. The role of squirrels in sustaining monkeypox virus transmission. Trop. Geogr. Med., 39: 115-122.
- Jenewari, F.O., 2019. Monkey pox in nigeria: epidemiology and prevention. Proceedings of the Problems and Prospects for the Development of Modern Medicine, GomSMU, Kharkiv, Ukrainian., 194–195.
- Kabuga, A.I., and El Zowalaty, M.E., 2019. A review of the monkeypox virus and a recent outbreak of skin rash disease in Nigeria. J. Med. Virol., 91: 533-540.
- Nasir, I.A., Dangana, A., Ojeamiren, I., and Emeribe, A.U., 2018. Reminiscing the recent incidence of monkeypox in Nigeria: Its ecologic-epidemiology and literature review. Port. Harcourt. Med. J., 12: 1.
- Sadeuh-Mba, S.A., Yonga, M.G., Els, M., Batejat, C., Eyangoh, S., Caro, V., and Njouom, R., 2019. Monkeypox virus phylogenetic similarities between a human case detected in Cameroon in 2018 and the 2017-2018 outbreak in Nigeria. Infect. Genet. Evol., 69: 8-11.
- Petersen, E., Kantele, A., Koopmans, M., Asogun, D., Yinka-Ogunleye, A., Ihekweazu, C., and Zumla, A., 2019. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect. Dis. Clin., 33: 1027-1043.
- Ugorji, C.V., Nworuh, B., Iwuoha, G., Abanobi., O.C., 2019. Factors associated with the practice of monkey pox preventive behaviours among health workers in Yenagoa LGA, Bayelsa state, Nigeria. IOSR. J. Nurs. Health. Sci., 8: 75–85.
- Chieloka, O.S., Amao, L.K., Akinrogbe, J.T., Iniobong, J.I., and Burga, J., 2019. Outbreak Investigation of Monkeypox in Akwa Ibom State: A Matched Case Control Study 14th-24th October 2019. East. Afr. j. public health. sci., 1: 37-44.
- Ellis, C.K., Carroll, D.S., Lash, R.R., Peterson, A.T., Damon, I.K., Malekani, J., and Formenty, P., 2012. Ecology and geography of human monkeypox case occurrences across Africa. J. Wildl. Dis., 48: 335-347.
- Ihekweazu, C., Yinka-Ogunleye, A., Lule, S., and Ibrahim, A., 2020. Importance of epidemiological research of monkeypox: is incidence increasing?. Expert. Rev. Anti. Infect. Ther., 18: 389-392.
- Vaughan, A., Aarons, E., Astbury, J., Balasegaram, S., Beadsworth, M., Beck, C. R., and Wilburn, J., 2018. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro. Surveill., 23: 1800509.
- Yinka-Ogunleye, A., Aruna, O., Ogoina, D., Aworabhi, N., Eteng, W., Badaru, S., and Ihekweazu, C., 2018. Reemergence of human monkeypox in Nigeria, 2017. Emerg. Infect. Dis., 24: 1149.
- Nolen, L.D., Osadebe, L., Katomba, J., Likofata, J., Mukadi, D., Monroe, B., and Reynolds, M.G., 2016. Extended human-to-human transmission during a monkeypox outbreak in the Democratic Republic of the Congo. Emerg. Infect. Dis., 22: 1014.
- Ogoina, D., Izibewule, J.H., Ogunleye, A., Ederiane, E., Anebonam, U., Neni, A., and Ihekweazu, C. 2019. The 2017 human monkeypox outbreak in Nigeria—report of outbreak experience and response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLoS. One., 14: e0214229.
- Magnus, P.V., Andersen, E.K., Petersen, K.B., and Birch‐Andersen, A., 1959. A Pox-like Disease in Cynomolgus Monkeys. Acta. Pathol. Microbiol. Scand., 46: 156–176.
- Mustaffa, F., Zaini, N.A., and Selvarajah, K., 2019. Review: An analysis of monkeypox disease and current scenario in Malaysia. Int. J. Res. Granthaalayah., 7: 82-87.
- World Health Organization (WHO)., 2020. Monkeypox.
- Dubois, M.E., and Slifka, M.K., 2008. Retrospective analysis of monkeypox infection. Emerg. Infect. Dis., 14: 592.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref