Research Article - Biomedical Research (2017) Volume 28, Issue 21
Curative effects of shikonin on allergic rhinitis in rats and the related mechanism
Ming Wang*, Gang Liu and Haiyan Li
Department of Otorhinolaryngology Head and Neck Surgery, Tianjin Huanhu Hospital, Tianjin, PR China
- *Corresponding Author:
- Ming Wang
Department of Otorhinolaryngology Head and Neck Surgery
Tianjin Huanhu Hospital, PR China
Accepted date: September 21, 2017
Abstract
This study aimed to investigate the curative effects of shikonin on Allergic Rhinitis (AR) in rats and explore the related mechanism. Fifty Sprague-Dawley rats were randomly divided into control group, model group, and low-, medium- and high-dose shikonin group, 10 rats in each group. In the later 4 groups, the AR model was constructed by intraperitoneal injection with ovalbumin suspension. In addition, the rats in low-, medium- and high-dose shikonin group received the intraperitoneal injection of shikonin with dose of 200, 400 and 600 μg/kg, respectively. On the 2, 4 and 6 d of experiment, the animal behavior score was measured. On the last day, the peripheral blood was taken, and the serum Interleukin-4 (IL-4), Interferon-γ (IFN-γ) and OVA-specific Immunoglobulin E (IgE) levels were determined using ELISA. The bilateral nasal mucosa tissues were taken. The Superoxide Dismutase (SOD), Malondialdehyde (MDA) and Glutathione Peroxidase (GSH-Px) levels in nasal mucosa tissue were determined, and the expression levels of T-bet and GATA-3 protein were detected. Results showed that, compared with model group, the AR features of rats in shikonin group was mitigated, the serum OVA-specific IgE and IL-4 level were decreased, and the serum IFN-γ level was increased. Compared with model group, the SOD and GSH-Px levels in nasal mucosa tissue in shikonin group was increased, and the MDA level was decreased. Compared with model group, the expression level of T-bet protein in nasal mucosa tissue in shikonin group was increased, and the GATA-3 expression level was decreased. In conclusion, shikonin can mitigate the AR in rats, which may be related to its anti-oxidative stress effects and regulation of T-bet and GATA-3 protein expression in nasal mucosa tissue.
Keywords
Shikonin, Allergic rhinitis, Oxidative stress, T-bet, GATA-3
Introduction
Allergic Rhinitis (AR) is an immediate hypersensitivity disease which occurs in the nasal mucosa and is mediated by Immunoglobulin E (IgE). AR is common in children and adults. It can affect the people's life quality, work and learning efficiency, and increase healthcare costs [1]. AR is closely associated with asthma. It is one of the risk factors of asthma. Both of AR and asthma belong to airway hyperresponsiveness diseases, and simultaneously exist in many patients. The active treatment of AR is one of the effective measures of preventing or reducing bronchial asthma [2]. The main features of AR are the nasal itching, sneezing, watery mucus and nasal congestion [3]. Some AR patients also have the hyposmia, hypogeusia, sleep disorders, headaches, and even anxiety and mood disorders [4,5]. The incidence of AR is increasing year by year, and it is an important problem that extensively affects the human health. Shikonin is a naphthoquinone compound extracted from the root of traditional Chinese herb Symphytum officinale. Studies show that, shikonin and its derivatives can inhibit the growth of bacteria and fungi, promote the tissue healing, inhibit the tumor cell proliferation, and promote the apoptosis [6-8]. In addition, it has the anti-inflammatory, antioxidant and anti-HIV activities [9-11], and involves complex cellular and molecular mechanisms. However, the effect of shikonin on AR has not been reported. This study investigated the curative effects of shikonin on allergic rhinitis in rats and explored the related mechanism. The objective was to provide a basis for clinical application of shikonin to treatment of AR.
Materials and Methods
Animal grouping and treatment
Fifty male SD (Beijing Vital River Laboratory Animal Technology Co., Ltd., Beijing, China) rats were randomly divided into control group, model group, and low-, mediumand high-dose shikonin group, 10 rats in each group. In the later 4 groups, the AR model of rats was constructed according to the reported methods [12]. In the basic immune stage, each rat was intraperitoneally injected with Ovalbumin (OVA) suspension (20 mg OVA and 30 mg aluminum hydroxide were dissolved in 1 ml of normal saline; Sigma-Aldrich Corp., MO, USA), once every other day, for a total of 6 times. In the control group, normal saline substituting OVA suspension was intraperitoneally injected. In the excitation stage, each modeled rat received the bilateral intranasal drop of 20-50 μl OVA normal saline solution (50 μg/L), once a day, a total of 6 times. The rats in control group received bilateral intranasal drop of normal saline instead of OVA solution. In low-, medium- and high-dose shikonin group, at 30 min before each excitation, 200, 400 and 600 μg/kg shikonin was used for intraperitoneal injection, respectively, once a day, a total of 6 times.
Determination of behavioral features of rats
On the 2, 4 and 6 d of experiment, the animal behavior features were determined within 30 min from excitation, which was based on the total volume of nasal discharge, number of sneezing and number of nose scratching. The scoring standards were as follows: i) nose scratching: incessant nose scratching, 3 points; frequent nose scratching (10-20 times/min), 2 points; mild nose scratching, 1 point; no nose scratching 0 point; ii) sneezing: ≥ 11 sneezes, 3 points; 4-10 sneezes, 2 points; 1-3 sneezes, 1 point; no sneeze, 0 point; iii) nasal discharge: face covered with nasal discharge, 3 points; nasal discharge beyond the nostril, 2 points; nasal discharge reaching the nostril, 1 point; no nasal discharge, 0 point.
Determination of serum levels of interleukin-4, interferon-γ and OVA-specific IgE
At the end of experiment, the peripheral blood of rats was taken by eyeball picking method. After standing for 2 h, the blood was centrifuged at 256X g for 10 min, and the supernatant was obtained. The serum levels of Interleukin-4 (IL-4), Interferon-γ (IFN-γ) and OVA-specific IgE were determined using ELISA. The procedure was according to the instructions of kits (Sigma-Aldrich Corp., MO, USA).
Determination of superoxide dismutase, glutathione peroxidase and malondialdehyde levels in nasal mucosa tissue
The rats were executed. The bilateral nasal mucosa tissues were taken. The 10% tissue homogenate was prepared using 4°C physiological saline. After refrigerating at 256X g and 4°C for 10 min, the supernatant was obtained for further use. The Superoxide Dismutase (SOD) level was detected by xanthine oxidase method [13]. The Glutathione Peroxidase (GSH-Px) level was measured by reduced glutathione depletion method [14]. The content of Malondialdehyde (MDA) was analysed by thiobarbituric acid colorimetric assay [15].
Determination of T-bet and GATA-3 protein expression in nasal mucosa tissue
The expressions of T-bet and GATA-3 protein in nasal mucosa tissue were determined using western blot assay. The nasal mucosa tissues were homogenized. The protein was extracted using RIPA lysis buffer (Santa Cruz Biotechnology (Shanghai) Co., Ltd., Shanghai, China). The protein concentration was determined by Coomassie brilliant blue method. 50 μg protein was used for the SDS-PAGE (Sigma-Aldrich Corp., MO, USA), then the separated protein was transferred to the PVDF membrane (Shanghai Sangon Biological Engineering Technology And Service Co., Ltd., Shanghai, China). After blocking using l% BSA (Fuzhou Maixin Biotechnology Development Co., Ltd., Fuzhou, China), the membranes were incubated with primary antibody overnight at 4°C, followed by washing with PBS. The horseradish peroxidase-labeled second antibody was added, followed by incubation at 37°C for 2 h. Visualization was accomplished by the enhanced chemiluminescence (ECL plus Western-blotting detection system, GE Healthcare Life Sciences, MA, USA). The intensity of bands was calculated with Image J 1.46 analysis software (European Molecular Biology Laboratory Inc., Oxford, UK). The primary and secondary antibodies were provided by Fuzhou Maixin Biotechnology Development Co., Ltd. (Fuzhou, China). β-actin (Sigma-Aldrich Corp., MO, USA) was used as the internal reference.
Statistical analysis
All statistical analysis was carried out using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). The data were presented as mean ± SD. The difference between two groups was analysed using one-way ANOVA with SNK-q test. p<0.05 was considered as statistically significant.
Results
Effect of shikonin on behavioral features of AR rats
After OVA stimulation, compared with the control group, the rats in the model group presented obvious AR features including nasal itching, sneezing and scratching face. Compared with the model group, the AR features in three shikonin groups were mitigated. In model and three shikonin groups, the behavioral score of rats was gradually increased, with the time prolonging in excitation stage. On the d 2, 4 and 6 of excitation stage, the behavioral score in model group was significantly higher than control group, respectively (p<0.05). Compared with model group, the behavioral scores in highdose shikonin group (d 2), middle- and high-dose shikonin group (d 4) and low-, middle- and high-dose shikonin group (d 6) were significantly decreased, respectively (p<0.05) (Table 1).
| Group | Day 2 | Day 4 | Day 6 |
|---|---|---|---|
| Control | 0.45 ± 0.04 | 0.61 ± 0.05 | 0.55 ± 0.06 |
| Model | 4.43 ± 0.86a | 6.12 ± 0.98a | 6.56 ± 1.02a |
| Low-dose shikonin | 4.36 ± 1.04a | 5.42 ± 0.72a | 5.62 ± 0.89ab |
| Middle-dose shikonin | 4.12 ± 0.56ac | 5.04 ± 0.81ab | 5.14 ± 1.01ab |
| High-dose shikonin | 3.34 ± 0.58abcd | 4.57 ± 0.62abc | 4.87 ± 0.87ab |
Table 1. Effect of shikonin on behavioral features of AR rats.
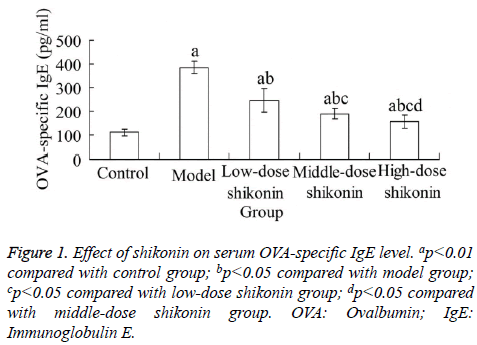
Effect of shikonin on serum OVA-specific IgE level
At the end of experiment, compared with the control group, the serum OVA-specific IgE level in model group was significantly increased, respectively (p<0.05). Compared with the model group, the serum OVA-specific IgE level in three shikonin groups were significantly decreased, respectively (p<0.05). In addition, there was significant difference between each two of three shikonin groups (p<0.05) (Figure 1).
Effect of shikonin on serum IL-4 and IFN-γ level
Compared with the control group, the serum IL-4 level in model group was significantly increased (p<0.05), and the serum IFN-γ level in model group was significantly decreased (p<0.05). Compared with the model group, the serum IL-4 levels in middle- and high-dose shikonin group were significantly decreased, respectively (p<0.05), and the serum IFN-γ level in these two groups were significantly increased, respectively (p<0.05) (Table 2).
| Group | IL-4 (pg/ml) | IFN-γ (pg/ml) |
|---|---|---|
| Control | 160.57 ± 22.17 | 423.28 ± 56.45 |
| Model | 280.17 ± 32.29a | 236.28 ± 45.11a |
| Low-dose shikonin | 300.21 ± 38.62a | 262.36 ± 33.83a |
| Middle-dose shikonin | 234.27 ± 38.82abc | 323.26 ± 51.09abc |
| High-dose shikonin | 201.57 ± 29.05abcd | 367.45 ± 49.27abc |
Table 2. Effect of shikonin on serum IL-4 and IFN-γ level.
Effect of shikonin on SOD, GSH-Px and MDA levels in nasal mucosa tissue
Compared with the control group, the SOD and GSH-Px levels in nasal mucosa tissue in model group were significantly decreased, respectively (p<0.05), and the content of MDA was significantly increased (p<0.05). Compared the model group, the SOD levels in middle- and high-dose shikonin group were significantly increased, respectively (p<0.05), In addition, the GSH-Px levels in low-, middle- and high-dose shikonin group were significantly increased, respectively (p<0.05), and the content of MDA in these three groups were significantly decreased, respectively (p<0.05) (Table 3).
| Group | SOD (U/mgprot) | GSH-Px(U/mgprot) | MDA (mmol/mgprot) |
|---|---|---|---|
| Control | 189.66 ± 21.34 | 140.01 ± 18.67 | 3.45 ± 0.46 |
| Model | 135.81 ± 15.71a | 84.21 ± 12.32a | 8.27 ± 1.01a |
| Low-dose shikonin | 143.24 ± 13.27a | 95.89 ± 11.93ab | 6.29 ± 0.78ab |
| Middle-dose shikonin | 153.37 ± 17.38abc | 115.67 ± 13.41abc | 5.34 ± 0.68abc |
| High-dose shikonin | 171.91 ± 11.21abd | 126.23 ± 16.44bc | 4.12 ± 0.72abcd |
Table 3. Effect of shikonin on SOD, GSH-Px and MDA levels in nasal mucosa tissue.
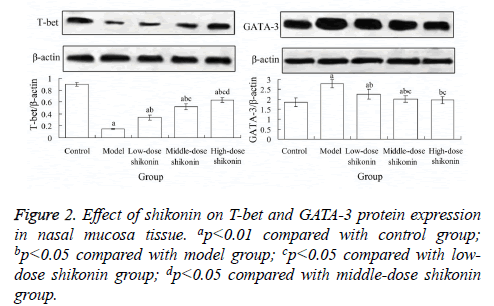
Effect of shikonin on T-bet and GATA-3 protein expression in nasal mucosa tissue
Compared with the control group, the expression level of T-bet protein in nasal mucosa tissue in model group was significantly decreased (p<0.05), and the expression level of GATA-3 protein was significantly increased (p<0.05). Compared with the model group, the expression level of T-bet protein in low-, middle- and high-dose shikonin group were significantly increased, respectively (p<0.05), and the expression level of GATA-3 protein in these three groups were significantly decreased, respectively (p<0.05) (Figure 2).
Discussion
At present, the treatments of AR mainly involve avoiding allergen, drug therapy and desensitization therapy, among which the drug therapy is the most common used way [1]. The commonly clinically used drugs for treatment of AR include antihistamines, nasal hormones, anti-leukotriene drugs, antiacetylcholine drugs, anti-congestion drugs, and traditional Chinese medicines [16-19]. However, so far there is no Chinese herbal extract with definite component for clinical first-line treatment of AR. This study established the AR model of rats using OVA stimulation, and applied shikonin to treatment of AR. Results showed that, after OVA stimulation, compared with the control group, the rats in the model group of presented obvious AR symptoms. Compared with the model group, the symptoms in shikonin group were mitigated. In addition, compared with the control group, the behavioral score and OVA-specific IgE level in model group were significantly increased (p<0.05), but those in shikonin group were significantly decreased, compared with the model group (p<0.05). This indicates that, the AR model of rats has been successfully constructed in this study, and the shikonin can obviously alleviate the OVA-induced AR.
IL-4 is a lymphokine produced by T helper (Th) cells. The effect of IL-4 on immune response is mainly its inhibition of cellular immunity and promotion of humoral immunity, especially the IgE reaction [20]. IL-4 is a specific inducer of IgE, and it regulates the release of a variety of inflammatory mediators from immune cells, resulting increased mucus secretion in nasal mucosain, increased vascular permeability and inflammatory cells infiltration [21]. IFN-γ is the cytokine secreted by Th1 cells. The main role of IFN-γ is the induction of B cells to produce IgG and IgM, and inhibition of IgE production. It can enhance Th1 cell immune response and inhibit Th2 cell responses [22]. It is found that, reducing the IL-4 level in the blood and increasing the IFN-γ level can alleviate the pathological lesion of AR in rats [23]. In this study, compared with the control group, the serum IL-4 level in model group was significantly increased, respectively (P<0.05), and the serum IFN-γ level in model group was significantly decreased (P<0.05). Compared with the model group, the serum IL-4 levels in middle- and high-dose shikonin group were significantly decreased, respectively (P<0.05), and the serum IFN-γ level in these two groups were significantly increased, respectively (P<0.05). This indicates that, the mechanism of shikonin alleviating AR may be related to its decreasing IL-4 level and increasing IFN-γ level in the body.
Oxidative stress is involved in many pathophysiological processes of the body. The cytotoxicity of reactive oxygen species can affect the cell function, thus causing progressive damage to body and aggravating the disease status [24]. Oxidative stress is also an important mechanism in the pathogenesis of AR. Oxidative stress and allergic reactions interact with each other, thus aggravating the symptoms of AR [25]. SOD, GSH-Px and MDA are the important indicators which reflect the oxidative stress degree in the body. Results of this study showed that, compared with the model group, the SOD levels in middle- and high-dose shikonin group were significantly increased, respectively (p<0.05), the GSH-Px levels in low-, middle- and high-dose shikonin group were significantly increased, respectively (p<0.05), and the content of MDA in 3 shikonin groups were significantly decreased, respectively. This indicates that, the shikonin has the antioxidative stress effects, which may be related to its role in alleviating AR.
It is found that, the mechanism of AR is related to Th1/Th2 imbalance in the body [26]. T-bet and GATA-3 are important transcription factors in the process of Th cell differentiation. Tbet can promote the differentiation of Th1 cells. The role of GATA-3 is contrary to T-bet. It regulates the differentiation of Th2 cells and secretion of Th2-type cytokines, thereby inhibiting the differentiation of Th1 cells. T-bet/GATA-3 balance determines the fate of T cells, and can be used as an important index to evaluate the Th1/Th2 imbalance in AR [27,28]. Results of this study showed that, compared with the model group, the expression level of T-bet protein in low-, middle- and high-dose shikonin group were significantly increased, respectively (p<0.05), and the expression level of GATA-3 protein in these three groups were significantly decreased, respectively (p<0.05). This indicates that, the role of shikonin in alleviating AR may be related to its regulation of Tbet/ GATA-3 signaling pathway in the body.
In conclusion, shikonin can mitigate the AR in rats, which may be related to its anti-oxidative stress effects and regulation of T-bet and GATA-3 protein expression in nasal mucosa tissue. This study has provided an experimental basis for the clinical application of shikonin to treatment of AR. However, whether there are other mechanisms of shikonin in alleviating AR should be further studied.
References
- Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet 2011; 378: 2112-2122.
- Bousquet J, Van Cauwenberge P, Khaltaev N, Aria Workshop Group, World Health Organization. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol 2001; 108: S147-334.
- Ratner P, Falqués M, Chuecos F, Esbri R, Gispert J, Peris F, Luria X, Rosales MJ. Meta-analysis of the efficacy of ebastine 20 mg compared to loratadine 10 mg and placebo in the symptomatic treatment of seasonal allergic rhinitis. Int Arch Allergy Immunol 2005; 138: 312-318.
- Cowart BJ, Flynn-Rodden K, McGeady SJ, Lowry LD. Hyposmia in allergic rhinitis. J Allergy Clin Immunol 1993; 91: 747-751.
- Craig TJ, Teets S, Lehman EB, Chinchilli VM, Zwillich C. Nasal congestion secondary to allergic rhinitis as a cause of sleep disturbance and daytime fatigue and the response to topical nasal corticosteroids. J Allergy Clin Immunol 1998; 101: 633-637.
- Andujar I, Ríos JL, Giner RM, Recio MC. Pharmacological properties of shikonin-a review of literature since 2002. Planta Med 2013; 79: 1685-1697.
- Wang R, Yin R, Zhou W, Xu D, Li S. Shikonin and its derivatives: a patent review. Expert Opin Ther Pat 2012; 22: 977-997.
- Chen X, Yang L, Oppenheim JJ, Howard MZ. Cellular pharmacology studies of shikonin derivatives. Phytother Res 2002; 16: 199-209.
- Tanaka S, Tajima M, Tsukada M, Tabata M. A comparative study on anti-inflammatory activities of the enantiomers, shikonin and alkannin. J Nat Prod 1986; 49: 466-469.
- Assimopoulou AN, Boskou D, Papageorgiou VP. Antioxidant activities of alkannin, shikonin and Alkanna tinctoria root extracts in oil substrates. Food Chem 2004; 87: 433-438.
- Chen X, Yang L, Zhang N, Turpin JA, Buckheit RW, Osterling C, Oppenheim JJ, Howard OM. Shikonin, a component of Chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1. Antimicrob Agents Chemother 2003; 47: 2810-2816.
- Tatar A, Yayla M, Kose D, Halici Z, Yoruk O, Polat E. The role of endothelin-1 and endothelin receptor antagonists in allergic rhinitis inflammation: ovalbumin-induced rat model. Rhinology 2016; 54: 266-272.
- Minami M, Yoshikawa H. A simplified assay method of superoxide dismutase activity for clinical use. Clin Chim Acta 1979; 92: 337-342.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967; 70: 158-169.
- Placer ZA, Cushman LL, Johnson BC. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem 1966; 16: 359-364.
- Wang XY, Lim-Jurado M, Prepageran N, Tantilipikorn P, Wang de Y. Treatment of allergic rhinitis and urticaria: a review of the newest antihistamine drug bilastine. Ther Clin Risk Manag 2016; 12: 585-597.
- Shao YY, Zhou YM, Hu M, Li JZ, Chen CJ, Wang YJ, Shi XY, Wang WJ, Zhang TT. The anti-allergic rhinitis effect of traditional Chinese medicine of Shenqi by regulating mast cell degranulation and Th1/Th2 cytokine balance. Molecules 2017; 22: E504.
- Wei J, Gerlich J, Genuneit J, Nowak D, Vogelberg C, von Mutius E, Radon K. Hormonal factors and incident asthma and allergic rhinitis during puberty in girls. Ann Allergy Asthma Immunol 2015; 115: 21-27.
- Cingi C, Muluk NB, Ipci K, Şahin E. Antileukotrienes in upper airway inflammatory diseases. Curr Allergy Asthma Rep 2015; 15: 64.
- Chatterjee S, Clark CE, Lugli E, Roederer M, Nutman TB. Filarial infection modulates the immune response to Mycobacterium tuberculosis through expansion of CD4+ IL-4 memory T cells. J Immunol 2015; 194: 2706-2714.
- Chai R, Liu B, Qi F. The significance of the levels of IL-4, IL-31 and TLSP in patients with asthma and/or rhinitis. Immunotherapy 2017; 9: 331-337.
- Yotsumoto S, Kakiuchi T, Aramaki Y. Enhancement of IFN-gamma production for Th1-cell therapy using negatively charged liposomes containing phosphatidylserine. Vaccine 2007; 25: 5256-5262.
- Li Y, Simons FE, Jay FT, HayGlass KT. Allergen-driven limiting dilution analysis of human IL-4 and IFN-gamma production in allergic rhinitis and clinically tolerant individuals. Int Immunol 1996; 8: 897-904.
- Xia XJ, Zhou YH, Shi K, Zhou J, Foyer CH, Yu JQ. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J Exp Bot 2015; 66: 2839-2856.
- Celik M, Tuncer A, Soyer OU, Saçkesen C, Tanju Besler H, Kalayci O. Oxidative stress in the airways of children with asthma and allergic rhinitis. Pediatr Allergy Immunol 2012; 23: 556-561.
- Luo Y, Deng Y, Tao Z, Chen S, Xiao B, Ren J, Chen Z, Han J, Kong Y, Xu Y, Deng M. Regulatory effect of microRNA-135a on the Th1/Th2 imbalance in a murine model of allergic rhinitis. Exp Ther Med 2014; 8: 1105-1110.
- Eifan AO, Furukido K, Dumitru A, Jacobson MR, Schmidt-Weber C, Banfield G, Durham SR, Nouri-Aria KT. Reduced T-bet in addition to enhanced STAT6 and GATA3 expressing T cells contribute to human allergen-induced late responses. Clin Exp Allergy 2012; 42: 891-900.
- Li K, Chen Y, Jiang R, Chen D, Wang H, Xiong W, Li D, Liu Z, Li X, Li J, Yuan K. Protective effects of astragaloside IV against ovalbumin-induced allergic rhinitis are mediated by T-box protein expressed in T cells/GATA-3 and forkhead box protein 3/retinoic acid-related orphan nuclear receptor γt. Mol Med Rep 2017; 16: 1207-1215.