Research Article - Biomedical Research (2017) Volume 28, Issue 17
Curative effect assessed with computed tomography perfusion imaging following intracranial and extracranial bypass for ischemic cerebrovascular disease
Man Gao1#, Yi Zhang1# and Jiaxin Chi2*
1Department of Neuroradiology, Tianjin Huanhu Hospital, Tianjin, PR China
2Department of Neurosurgery, Tianjin Huanhu Hospital, Tianjin, PR China
#These authors have equally contributed to this study.
- *Corresponding Author:
- Jiaxin Chi
Department of Neurosurgery
Tianjin Huanhu Hospital
Tianjin, PR China
Accepted date: August 04, 2017
Abstract
By utilizing Computed Tomography (CT) perfusion technology, we analyzed hemodynamic characteristics before and after intracranial and extracranial vascular anastomosis for ischemic cerebrovascular disease and evaluated therapeutic efficacy. Fifty-seven patients with ischemic cerebrovascular disease underwent intracranial and extracranial vascular anastomoses. CT perfusion was evaluated before and after anastomosis. The preoperative diagnoses included Transient Ischemic Attack (TIA), Reversible Ischemic Neurological Deficit (RIND), and atypical symptoms of cerebral ischemia. Evaluation of middle cerebral artery distribution and cerebral watershed area indicated obvious delay in CT perfusion. Regional cerebral blood flow was slightly reduced or normal, and perfusion in the cerebral watershed area after anastomosis recovered to normal. The area of perfusion delay was obviously reduced after anastomosis. CT perfusion indicated improvement of 84.2%. CT perfusion technology can accurately evaluate the hemodynamic status of unilateral anterior circulation arterial stenosis, and surgical treatment can improve this hemodynamic barrier. The curative effect was objectively and accurately evaluated using CT perfusion technology.
Keywords
Ischemic cerebral vascular disease, CT perfusion, Intracranial and extracranial vascular anastomosis, Middle cerebral artery stenosis
Introduction
Carotid artery stenosis or occlusion is common, involves the internal carotid and middle cerebral arteries, and is frequently caused by atherosclerosis or arteritis [1-3]. Embolic cerebral infarction can occur because of detached plaque or thrombus, and can even result from decreased blood velocity, which acts as a hemodynamic barrier. Timely blood reperfusion can reduce the risk of infarction in patients with carotid artery stenosis [4-6]. Therefore, before surgical intervention, evaluation should be performed to determine whether cerebral ischemia will respond to blood reperfusion after anastomosis. Evaluation of cerebral blood flow perfusion can accurately reflect the blood supply of brain tissue, thereby providing an important reference for design of a clinical treatment plan [7-10]. In this research, we retrospectively analyzed the efficacy of anastomosis in 57 patients with unilateral middle cerebral artery stenosis who underwent surgery; the patients were evaluated before and after anastomosis, using 64-slice Computed Tomography (CT) and Perfusion CT (PCT).
Methods
Clinical data
We retrospectively analyzed perfusion images in 57 patients with cerebral ischemia before and after middle cerebral artery anastomosis, between July 2008 and December 2013. The patients included 39 men and 18 women, with mean age 54.6 ± 5.1 y (range, 46-70 y). All patients had a history of cerebral ischemia, 40 had a Transient Ischemic Attack (TIA), 8 had a Reversible Ischemic Neurological Deficit (RIND), and 9 had a cerebral infarction. Cerebral angiography showed that all patients had some degree of internal carotid artery stenosis and unilateral middle cerebral artery stenosis. Agitated or uncooperative patients with impaired consciousness were excluded. All patients underwent 64-slice spiral CT perfusion imaging 1 d before and 5 d after anastomosis. All patients underwent digital subtraction angiography or 64-slice spiral CT perfusion imaging (CT angiography CTA)) before anastomosis, and were found to have cerebrovascular disease due to unilateral middle cerebral artery stenosis. Informed consent was obtained from the patients or their families. After examination, the patients underwent superficial temporal artery-middle cerebral artery anastomosis.
A GE Light Speed CT unit was used for all imaging. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Tianjin Huanhu Hospital. Our study will issue an informed consent letter to each patient and family member for their approval and signature confirmation.
Image and data processing
The frontal, central, and posterior white matter in the half-oval central layer was assessed for Cerebral Blood Flow (CBF), Cerebral Blood Volume (CBV), and Mean Transit Time (MTT) in the bilateral internal carotid artery supply area. Results for the paretic side were compared with the computer-generated relative values of rCBF, rCBV and rMTT at corresponding locations. Each Region of Interest (ROI) measured about 100 mm2. When obtaining the ROI, one should confirm the infarct area and large blood vessel supply region. Bilateral absolute values before anastomosis and relative values before and after anastomosis and recovery of perfusion should be compared in the ischemic areas.
Statistical analysis
The measurement data are shown as ͞x ± s. Statistical analysis was performed using SPSS 13.0 software. The measurement data were evaluated with the paired t-test and analysis of variance. A P value<0.05 was considered statistically significant.
Results
PCT evaluation of intracranial status before anastomosis
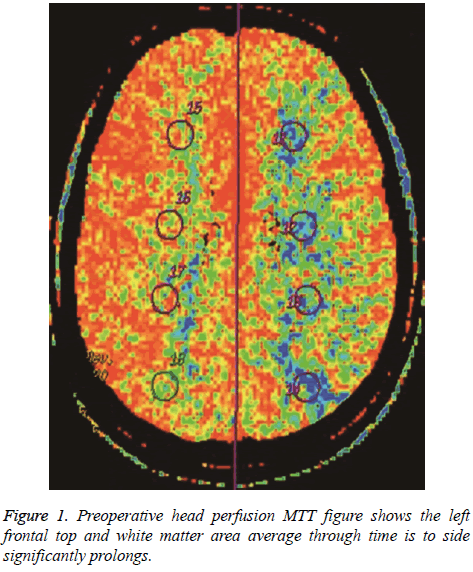
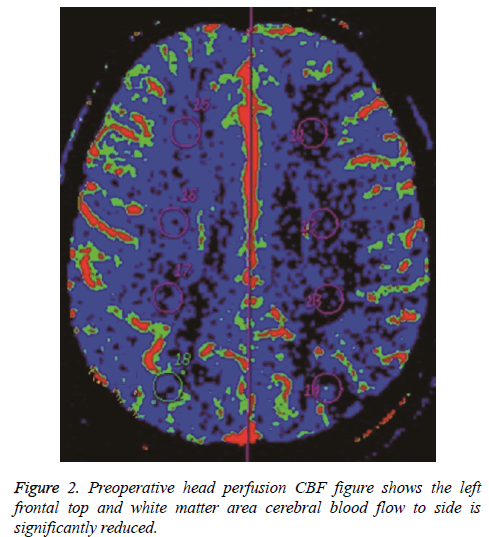
Before surgery, rCBF and MTT were determined for the arterial distribution and watershed areas on the paretic and unaffected sides. Analysis of variance showed the following: (1) There was no significant difference in regional cerebral blood flow in all patients (P>0.05), indicating no obvious abnormality in flow on the paretic side; (2) MTT in the middle cerebral artery distribution on the paretic side was obviously delayed (P<0.05), indicating perfusion delay in the two areas (Figures 1 and 2).
PCT evaluation of intracranial status after anastomosis
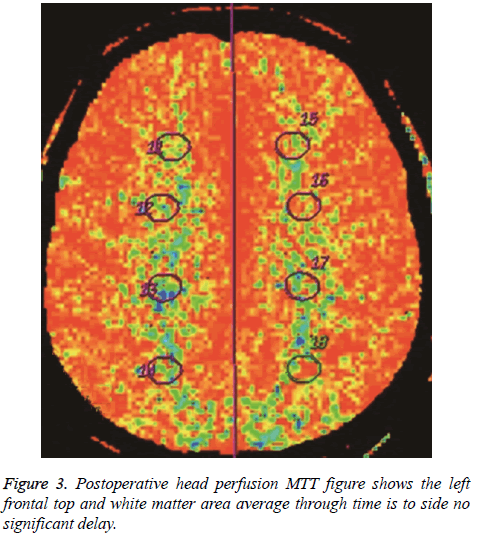
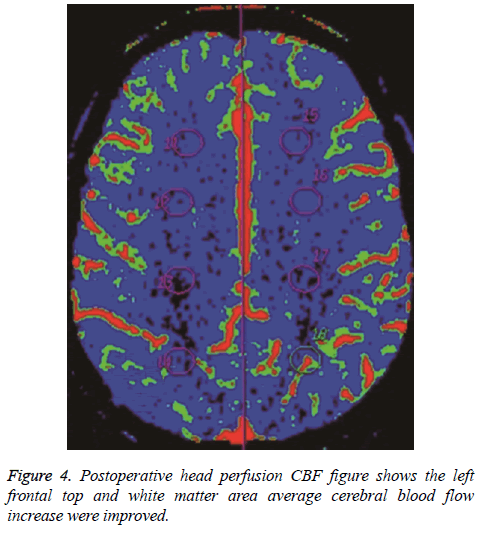
The hemodynamic index was obtained before anastomosis in all patients. The paretic side was compared with the unaffected side. Analysis of variance showed the following: (1) regional cerebral blood flow remained normal (P>0.05); (2) MTT in the watershed area after anastomosis was similar to MTT in the unaffected side, without statistical significance (P>0.05), but MTT in the middle cerebral artery distribution was relatively prolonged when compared with MTT in the unaffected side (P=0.01), indicating lack of recovery to the normal state.
Comparison of changes in PCT hemodynamic parameters
In all patients, PCT parameters were compared before and after anastomosis and paired examination and analysis were performed (Figures 3 and 4). The results were as follows: (1) There was no obvious difference in regional cerebral blood flow before and after anastomosis (P>0.05); (2) MTT in the middle cerebral artery distribution was shorter (P=0.021) after anastomosis. Therefore, surgery improved the delay in MTT. After anastomosis, blood flow improved in 48 of the 57 patients, but no MTT improvement was observed in 9 patients. MTT was significantly shorter after anastomosis (P<0.05). Color-coded images showed pathological changes more clearly for MTT than for CBF. The extent of cerebral ischemia was more defined. CT follow-up after anastomosis showed a small amount of bleeding in the ischemic area in 6 cases. Retrospective analysis of CTA before anastomosis showed that the mean rCBV was greater in the bleeding area in 6 patients with hemorrhage.
Discussion
Middle cerebral artery stenosis or occlusion is an important cause of ischemic cerebrovascular disease. Patients with significant occlusion, who do not respond to conservative treatment, should be considered for extracranial and intracranial arterial bypass or intracranial vascular stenting. Research has long been conducted on treatment of middle cerebral artery stenosis or occlusion by performing extracranial and intracranial arterial bypass. Klopfenstein et al. reported a series of experiments regarding treatment of middle cerebral artery stenosis or occlusion with extracranial and intracranial arterial bypass [11,12]. In our study, 55 patients were treated with superficial temporal artery-middle cerebral artery bypass. The remaining 2 were treated with occipital artery-middle cerebral artery bypass. The vascular anastomoses were satisfactory on follow-up. No further ischemic symptoms were observed in 88% of the patients. Of these patients, all 57 were followed up for 10 months. Neurological function improved in 50 patients (88%). Therefore, this research demonstrated the effectiveness of extracranial and intracranial arterial bypass in treating ischemic cerebrovascular disease caused by cerebral artery stenosis or occlusion.
The use of intracranial and extracranial vascular anastomosis in the treatment of carotid artery stenosis has increased. The efficacy is indirectly evaluated by improvement in clinical symptoms. This method is limited by subjectivity. Therefore, a more objective method should be used to evaluate treatment efficacy by performing an evaluation of microvascular cerebral blood flow. The clinical application of PCT has fulfilled this need. With the emergence of multilayer spiral CT and improvements in computer processing technology, the assessment of perfusion scans and parameters has received increasing attention. PCT can evaluate the blood supply in local brain tissue and enables semi-quantitative analysis of the extent of ischemia.
Meanwhile, compared with nuclear and magnetic resonance perfusion imaging, CT perfusion imaging provides goodquality images, accurate measurements, and good reproducibility. Although no obvious clinical symptoms were observed, the extent of restricted CBF in the middle cerebral artery distribution was slightly reduced, possibly because CBF was still adapting to changes during progression to ischemia. Research shows that when the blood flow rate in brain tissue is maintained at 46 ± 24 ml/100 g/min, patients will not show clinical symptoms of cerebral ischemia [13].
However, constant low perfusion in brain tissue will lead to long-term ischemic restriction of brain cells, resulting in neurological symptoms, including depression and cognitive deficits. The foundation of treatment lies in recovery of cerebral blood flow perfusion. Timely intracranial and extracranial vascular anastomoses can improve or even reverse symptoms [14,15].
In addition to the slight decrease in CBF, this research showed that MTT in middle cerebral artery stenosis was significantly prolonged. MTT can more accurately reflect the extent of ischemia and effect on brain tissue because MTT is negatively correlated with cerebral perfusion pressure [16].
According to the formula CBF=CBV/MTT, CBF is inversely proportional to MTT and proportional to CBV. In the early stages of ischemia caused by arterial stenosis, the decrease in CBF perfusion and impairment of cerebrovascular capacity leads to prolongation of MTT. Therefore, CBF remains within normal limits. When CBF perfusion is reduced to a degree that surpasses cerebrovascular capacity, CBF will be reduced [17]. Therefore, MTT is an early and sensitive indicator of impaired CBF perfusion [18]. Meanwhile, vascular expansion can lead to an increase in CBV. CBV will decrease when stenosis is increased, and collateral circulation cannot compensate for the deficit. As a result, CBV cannot be used for evaluation of early stages of ischemia.
Although CBV cannot reflect acute ischemia in brain tissue, the rCBV in the bleeding area in 6 patients was greater than average. This was caused by excessive vascular expansion caused by persistent ischemia. When blood circulates, the active oxygen and Ca2+ released during perfusion can damage the endothelium, which leads to vascular permeability or even microvascular rupture [19]. Therefore, high CBV in the central area of pathological change could be used as an index of the severity of ischemia and can predict complications and bleeding after surgery.
As a common method of evaluating local cerebral blood flow, CT is used to measure the perfusion value of CBF and other parameters before and after surgery. In practice, there are many advantages in the evaluation of CBF with CT perfusion, which can be performed immediately following regular CT examination. The method is easy to perform and master. The scanning and image processing time are relatively short. By using perfusion software for processing, image data after dynamic scanning can be rapidly transformed into several parameters. Therefore, the location of cerebral ischemia at an early stage and the extent of pathology can be determined. However, this technique makes measurement of cerebral blood flow in the cerebellum or brainstem difficult and is associated with increased radiation exposure [20]. Despite some deficiencies, PCT is still considered an effective method for examining CBF perfusion in patients with middle cerebral artery stenosis. Quantitative analysis can be used to objectively evaluate the treatment efficacy of middle cerebral artery anastomosis. CBF perfusion is used for routine evaluation of patients with suspected cerebral ischemia and for evaluation of circulation after treatment.
Conflicts of Interest
All of the authors declare that they have no conflicts of interest regarding this paper.
References
- Devlin TG, Phade SV, Hutson RK, Fugate MW, Major GR 2nd, Albers GW, Sirelkhatim AA, Sapkota BL, Quartfordt SD, Baxter BW. Computed tomography perfusion imaging in the selection of acute stroke patients to undergo emergent carotid endarterectomy. Ann Vasc Surg 2015; 29: 125.
- Cohen JE, Gomori M, Rajz G, Moscovici S, Leker RR, Rosenberg S, Itshayek E. Emergent stent-assisted angioplasty of extracranial internal carotid artery and intracranial stent-based thrombectomy in acute tandem occlusive disease: technical considerations. J Neurointerv Surg 2013; 5: 440-446.
- Dalyai RT, Chalouhi N, Singhal S, Jabbour P, Gonzalez LF, Dumont AS, Rosenwasser R, Ghobrial G, Tjoumakaris SI. Stent-assisted endovascular recanalization of extracranial internal carotid artery occlusion in acute ischemic stroke. World Neurosurg 2013; 79: 143-148.
- Nagaki T, Sato K, Yoshida T, Yoshimoto Y. Benefit of carotid endarterectomy for symptomatic and asymptomatic severe carotid artery stenosis: a Markov model based on data from randomized controlled trials. J Neurosurg 2009; 111: 970-977.
- Wintermark M, Thiran JP, Maeder P, Schnyder P, Meuli R. Simultaneous measurement of regional cerebral blood flow by perfusion CT and stable xenon CT: a validation study. AJNR Am J Neuroradiol 2001; 22: 905-914.
- Mlekusch W, Mlekusch I, Minar E, Haumer M, Kopp CW, Ahmadi R, Lehrner J, Schillinger M. Is there improvement of “vascular depression” after carotid artery stent placement? Radiol 2006; 240: 508-514.
- Mishra A, Stockley H, Goddard T, Sonwalker H, Wuppalapati S, Patankar T. Emergent extracranial internal carotid artery stenting and mechanical thrombectomy in acute ischaemic stroke. Interv Neuroradiol 2015; 21: 205-214.
- Leker RR, Eichel R, Keigler G, Gomori JM, Cohen JE. Occlusion site does not impact outcome in patients with carotid stroke undergoing endovascular reperfusion. Int J Stroke 2015; 10: 560-564.
- Yoon W, Kim SK, Park MS, Kim BC, Kang HK. Endovascular treatment and the outcomes of atherosclerotic intracranial stenosis in patients with hyperacute stroke. Neurosurg 2015; 76: 680-686.
- Pacheco FT, Littig IA, Gagliardi RJ, Rocha AJ. Multi-detector computed tomography angiography in clinically suspected hyperacute ischemic stroke in the anterior circulation: an etiological workup in a cohort of Brazilian patients. Arq Neuropsiquiatr 2015; 73: 408-414.
- Klopfenstein JD, Ponce FA, Kim LJ, Albuquerque FC, Nakaji P, Spetzler RF. Middle cerebral artery stenosis: endovascular and surgical options. Skull Base 2005; 15: 175-189.
- Andrews BT, Chater NL, Weinstein PR. Extra cranial intracranial bypass for middle cerebral artery stenos is and occlusion. Operative results in 65 cases. J Neurosurg 2015; 62: 831-838.
- Abdo M, Krayenbuhl N, Isolan GR, Krisht AF. Cerebral revascularization: part I: indications and evaluation. Contemp Neurosurg 2006; 28: 1-7.
- Garrett MC, Komotar RJ, Starke RM, Merkow MB, Otten ML, Sciacca RR, Connolly ES. The efficacy of direct extracranial-intracranial bypass in the treatment of symptomatic hemodynamic failure secondary to athero-occlusive disease: a systematic review. Clin Neurol Neurosurg 2009; 111: 319-326.
- Derdeyn CP, Grubb RL, Powers WJ. Cerebral hemodynamic impairment: methods of measurement and association with stroke risk. Neurol 1999; 53: 251-259.
- Wiesmann M, Berg S, Bohner G, Klingebiel R, Schöpf V, Stoeckelhuber BM, Yousry I, Linn J, Missler U. Dose reduction in dynamic perfusion CT of the brain: effects of the scan frequency on measurements of cerebral blood flow, cerebral blood volume, and mean transit time. Eur Radio 2008; 18: 2967-2974.
- Wintermark M, Flanders AE, Velthuis B, Meuli R, van Leeuwen M, Goldsher D, Pineda C, Serena J, van der Schaaf I, Waaijer A, Anderson J, Nesbit G, Gabriely I, Medina V, Quiles A, Pohlman S, Quist M, Schnyder P, Bogousslavsky J, Dillon WP, Pedraza S. Perfusion-CT assessment of infarct core and penumbra: receiver opcrating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 2006; 37: 979-985.
- Frantseva MV, Carlen PL, Perez Velazquez JL. Dynamics of intracellular calcium and free radical production during ischemia in pyramidal neurons. Free Radic Biol Med 2001; 31: 1216-1227.
- Gaudiello F, Colangelo V, Bolacchi F, Melis M, Gandini R, Garaci FG, Cozzolino V, Floris R, Simonetti G. Sixty-four-section CT cerebral perfusion evaluation in patients with carotid artery stenosis before and after stenting with a cerebral protection device. ANJR Am J Neuroradiol 2008; 29: 919-923.
- Hansen CK, Christensen A, Ovesen C, Havsteen I, Christensen H. Stroke severity and incidence of acute large vessel occlusions in patients with hyper-acute cerebral ischemia: results from a prospective cohort study based on CT-angiography (CTA). Int J Stroke 2015; 10: 336-342.