Research Article - Biomedical Research (2017) Volume 28, Issue 21
Cost-effectiveness of image-based surveillance for hepatocellular carcinoma in cirrhotic patients: a systematic review
Zhengping Xiong1 and Fang Huang2*
1Department of Interventional Radiology, the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, PR China
2Department of Infection Disease, the Third Xiangya Hospital of Central South University, Changsha, PR China
- *Corresponding Author:
- Fang Huang
Department of Infection Disease
The Third Xiangya Hospital of Central South University
Changsha, PR China
Accepted date: September 06, 2017
Abstract
This study aims to summarize cost-effectiveness analyses assessing image-based surveillance for Hepatocellular Carcinoma (HCC) in cirrhotic patients. Data was collected from main medical databases up to August 2016, to identify eligible studies assessing cost-effectiveness of HCC surveillance in cirrhotic patients. The included studies were reviewed to extract information on study design, surveillance strategies, model parameters, data sources of model variables, and results of base case analysis. Base case Incremental Cost-Effectiveness Ratio (ICER) per life year was adjusted to the 2015 currency value and presented as a ratio to the 2015 Gross Domestic Product (GDP) per capita for comparisons across countries. Simple linear regression analyses were performed to assess the impact of model variables on adjusted ICER per Quality Adjusted Life Year (QALY). Twelve studies from 8 countries were identified. When compared to no surveillance, the ICERs per life year associated with image-based surveillance were ranked by semi-annual Ultrasound (US) (0.16 GDP per capita), semiannual contrast-enhanced US (0.17 GDP per capita), annual US plus Alpha-Fetoprotein (AFP) (0.54 GDP per capita), semi-annual computed tomography plus AFP (0.60 GDP per capita), and semi-annual US plus AFP (0.63 GDP per capital). Semi-annual surveillance (coefficient 1.919, P=0.002) and annual mortality of decompensated cirrhosis (coefficient 13.762, P<0.001) were significantly associated with increased ICERs per QALY for image-based surveillance. Semi-annual US was likely the most costeffective image-based surveillance for HCC in cirrhotic patients. The cost-effectiveness of HCC surveillance was highly sensitive to surveillance frequency and mortality of decompensated cirrhosis.
Keywords
Incremental cost-effectiveness ratio (ICER), Liver cancer, Cirrhosis, Screening, Quality adjusted life year (QALY)
Introduction
Highly prevalent chronic viral hepatitis and limited access to effective antiviral therapy in developing countries and increasing incidence of hepatitis C-related cirrhosis in developed countries make Hepatocellular Carcinoma (HCC) continue to be a major global health problem and cause over half a million deaths globally per annum [1-3]. HCC is a curable disease when the tumor stage is detected early enough. Current curative therapy, including surgical resection, ablation, or liver transplantation could significantly improve the 5-y survival rate from 40% to 70% in patients with HCC smaller than 3 cm [4]. Thus, HCC surveillance in at-risk patients has been advocated and recommended by clinical practices guidelines for early detection of HCC [5-7].
Image-based methods are often recommended for HCC surveillance because they do not involve any invasive procedures and have relatively higher sensitivity and specificity to detect small HCC than other non-invasive methods [8-12]. Several studies have assessed the costeffectiveness of image-based HCC surveillance using a Markov model approach but it is challenging to generalize the generated economic evidence because of the differences in sensitivity and specificity of image-based techniques, time intervals between surveillance tests, surveillance population, patterns of care, cost perspective, model structure, and model variable estimates [13-37]. Thus, we conducted this study to systematically review the published cost-effectiveness analyses assessing image-based HCC surveillance and to explore how differences across those studies affect the cost-effectiveness of HCC surveillance.
Materials and Methods
This study was designed to systematically review the published cost-effectiveness analyses assessing image-based HCC surveillance in cirrhotic patients, irrespective of their etiology. The image-based techniques in our study included Ultrasound (US), Enhanced Contrast US (ECUS), Computed Tomography (CT), and Magnetic Resonance Imaging (MRI). This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Central South University. Written informed consent was obtained from all participants.
Identifying eligible studies
The medical databases searched in our study included MEDLINE, EMBASE, ISI Web of Science, and The Cochrane Library. The search period was set from January 1, 1990 to August 10, 2016. The search strategies were developed by combining key words for cost-effectiveness (costeffectiveness, cost-utility, cost-benefit, cost-minimization, incremental cost-effectiveness ratio, incremental cost-utility ratio, Incremental Cost-Effectiveness Ratio (ICER), Incremental Cost-Utility Ratio (ICUR), Markov, Willingness- To-Pay (WTP), Net Monetary Benefit (NMB)), image techniques used for HCC diagnosis (ultrasound, US, contrast enhanced ultrasound, contrast-enhanced ultrasonography (CEUS), computed tomography (CT), magnetic resonance imaging (MRI), HCC, liver cancer, or hepatoma), surveillance (detection, screening, screen, surveillance, or case finding), and cirrhosis (cirrhosis, cirrhotic, or cirrhotics). We also searched the proceedings of conferences related to liver diseases (the American Association for the Study of Liver Diseases (AASLD) and European Association For The Study Of The Liver (EASL)) or health economics (ISPOR, SMDM, HTAi, and iHTA) from 2011 to 2016 for any eligible studies that had not been fully published prior to the ending date of our literature search. The identified references from each searched database were pooled and cleaned by deleting duplicated records. The title and abstracts of the identified references were reviewed to identify references for further eligibility assessment by reviewing their full publication text. We included original studies comparing an image-based HCC surveillance strategy versus no surveillance or another HCC surveillance strategy for both health benefits and costs in cirrhotic patients, irrespective of their etiology. We excluded studies published in a language other than English and review articles, commentary, letters, or other publications, which were not original studies performing cost-effectiveness analysis.
Data extraction
We reviewed the full publication text of the eligible studies to extract the information on study design, HCC surveillance strategy, surveillance population, model approach, model structure, data sources for model variables, model assumptions, and the results of base case cost-effectiveness analysis. We extracted the time horizon, cost perspective, types of outcome measure for health benefits and costs, annual rates applied to discount benefits and/or costs, and model approach. The HCC surveillance strategies assessed in the cost-effectiveness analyses were reviewed to extract the type of image test and surveillance frequency. The surveillance populations in the cost-effectiveness analyses were identified to extract their baseline characteristics including age, gender, liver disease severity, and etiology of cirrhosis. If a Markov model was used to perform the cost-effectiveness analysis, we also extracted model cycle length, model assumptions, and model variable estimates. Additionally, the source references used to estimate model variables were traced and the original data in the source references were extracted for assessing uncertainty associated with model variable estimates. The type of currency and the currency year used in the cost-effectiveness analyses were extracted for cost adjustment in our study’s data analyses. Finally, the results of base case cost-effectiveness analyses for the assessed image-based HCC surveillance, usually presented by incremental cost-effectiveness ratios (ICER), were extracted.
Data analysis
Descriptive statistical methods were used to summarize the collected data for study characteristics, characteristics of HCC surveillance populations, model structures, and source references of model variable estimates. We performed a singlearm meta-analysis using the original data collected from source references of model probability and utility variables to demonstrate the current data gap in analyses assessing the costeffectiveness of HCC surveillance. The model cost variables were adjusted to the 2015 currency value using the country’s historic inflation rates and converted to US$ using the exchange rate on Dec 31, 2015. The base case ICERs associated with image-based HCC surveillance strategies were adjusted to the 2015 currency value using the country’s historic inflation rate and divided by the country’s 2015 GDP per capita in order to compare image-based HCC surveillance strategies across countries. Simple linear regression analyses were performed using HCV surveillance strategies and model estimates as independent variables to explore their impact on the cost-effectiveness of image-based surveillance indicated by the base case ICER per quality adjusted life year (QALY). SAS 9.2 was used to perform the data analyses described above and statistical significance defined in our study was a two-sided P value less than 0.05.
Results
The search of included medical databases and conference proceedings using the developed search strategies identified 253 references and 230 references were excluded after reading their titles and abstracts. The eligibility of the remaining 23 references was further assessed by reviewing their full publication text [13-37]. After excluding 2 reviews, 2 survey studies, 2 cohort studies, 3 cost studies [27,29], 1 model study, and 1 utility study, 12 studies meeting the inclusion criteria were included for data extraction and data analysis [14,15,18,20,23,25-27,29,31,32,34-36].
Characteristics of included studies
The included 12 studies comprised 11 full publications and 1 abstract that performed 8 cost-utility analyses and 5 cost-effectiveness analyses assessing HCC surveillance using US (5 studies), a combination of US and AFP (10 studies), a combination of CT and AFP (2 studies), CEUS (1 study), CT (1 study), MRI (1 study), or the combination of MRI and AFP (1 study) per 3 months (1 study), 6 months (11 studies), 10 months (1 study), or 12 months (6 studies) in cirrhotic patients with chronic hepatitis C (3 studies), chronic hepatitis B (2 studies), mixed chronic hepatitis B and C (1 study), or mixed etiology (6 studies) in 8 countries, which were the United States (4 studies), the United Kingdom (1 study), Italy (1 study), Switzerland (1 study), Japan (2 studies), Singapore (1 study), Taiwan (1 study), and Thailand (1 study) [14,15,18,20,23,25,26,31,32,34-36].
The 12 studies developed Markov models to assess both the health benefits and costs associated with image-based HCC surveillance from a social (3 studies) or payer’s perspective (9 studies) in a time horizon of 10 y (1 study), 20 y (2 studies), 25 y (2 studies), more than 30 y (3 studies), or unspecified (2 studies). Of the Markov models in these 12 studies, 2 had a 1- month cycle length, 8 had a 6-month cycle length, and 2 had a 1-y cycle length. Over half of the Markov models contained the following health states: death, HCC terminal stage, compensated cirrhosis with or without HCC, decompensated cirrhosis with or without HCC, HCC prior to terminal stage, and HCC post resection. However, very few models took into account HCC recurrence and other curative treatments for HCC, such as liver transplantation and ablation.
Model variable estimates based on source references
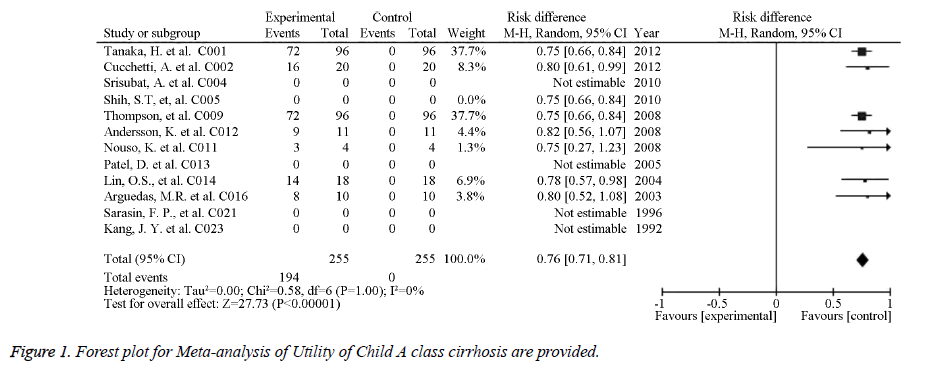
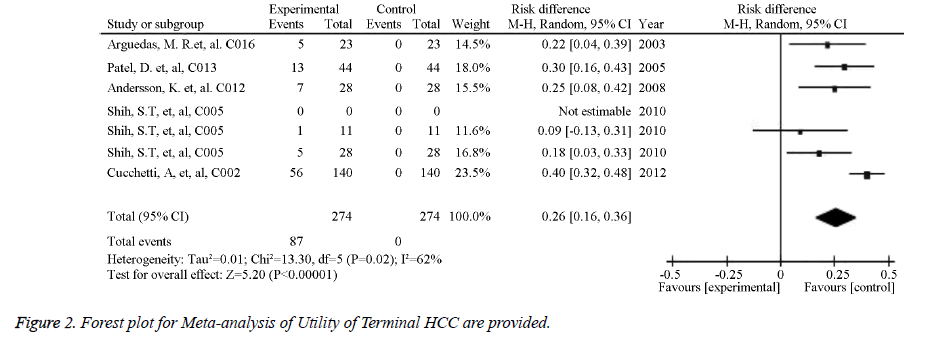
The source references used to estimate the probability and utility variables in the Markov models were identified for pooled estimates. Of the studied image techniques used in HCC surveillance strategies, US had the largest number of source references used to estimate its sensitivity and specificity for detecting 2 to 5 cm HCCs [30]. Model variables for the sensitivity and specificity of the imaging methods used for HCC screening in cirrhotic patients are provided in Table 1. The numbers of identified source references used to estimate the sensitivity and specificity of CT, US plus AFP, MRI, and CEUS for detecting HCC less than 3 cm were 12, 11, 5, and 1, respectively. The pooled estimations based on the source references for the sensitivity and specificity ranged from 0.645 for US to 0.900 for CEUS and from 0.859 for US plus AFP to 0.985 for CEUS, respectively. Meta-analysis of Utility of Child A class cirrhosis-Forest plot and Meta-analysis of Utility of Terminal HCC-Forest plot are provided in Figures 1 and 2. Other probability variables with 10 or more source references included annual risk of HCC, annual mortality of advanced HCC, first year mortality after HCC resection, annual mortality of compensated cirrhosis, annual mortality of decompensated cirrhosis, annual transition probability from compensated cirrhosis to decompensated cirrhosis, annual transition probability from small HCC to large HCC, and risk of HCC recurrence. A summary of the model variables for natural history of cirrhosis and HCC used in assessing the costeffectiveness of HCC screening in cirrhotic patients is provided in Table 2. Health states with 10 or more source references for their utility estimates included decompensated cirrhosis, compensated cirrhosis, liver transplantation, HCC late stage, and HCC prior to late stage. A summary of the utility variables used in the included studies assessing cost-effectiveness of screening for HCC in cirrhotic patients is provided in Table 3.
| Variable name | Pooled estimation | Data sources (number of references) | |||
|---|---|---|---|---|---|
| Tumor size | Mean | 95% CI lower limit | 95% upper limit | ||
| Specificity of CEUS | <3 cm | 0.95 | 0.8 | 0.95 | 1 |
| Sensitivity of CT | <3 cm | 0.95 | 0.9 | 1 | 12 |
| Specificity of CT | <3 cm | 0.91 | 0.86 | 0.96 | 12 |
| Specificity of US | 2-5 cm | 0.91 | 0.86 | 0.96 | 14 |
| Sensitivity of CEUS | <3 cm | 0.9 | 0.8 | 0.95 | 1 |
| Specificity of MRI | 0.5-2.0 cm | 0.9 | 0.8 | 0.96 | 5 |
| Sensitivity of AFP+US | <3 cm | 0.85 | 0.71 | 0.99 | 11 |
| Sensitivity of MRI | 0.5-2.0 cm | 0.85 | 0.55 | 0.91 | 5 |
| Specificity of AFP | <3 cm | 0.83 | 0.76 | 0.9 | 15 |
| Specificity of AFP+US | <3 cm | 0.81 | 0.73 | 0.88 | 8 |
| Sensitivity of US | 2-5 cm | 0.64 | 0.52 | 0.76 | 30 |
| Sensitivity of AFP | <3 cm | 0.58 | 0.43 | 0.72 | 18 |
Table 1. Summary of model variables for sensitivity and specificity of image methods used for HCC screening in cirrhotic patients.
| Variable name (annual transition probability) | Pooled estimation | Data sources (number of references) | ||
|---|---|---|---|---|
| Mean | 95% CI lower limit | 95% upper limit | ||
| Complication rate of liver biopsy | 0.01 | 0 | 0.1 | 4 |
| Mortality of liver transplant for HCC (first year) | 0.1 | 0.05 | 0.21 | 3 |
| Mortality of liver transplant for HCC (subsequent year) | 0.04 | 0.02 | 0.15 | 3 |
| Mortality of advanced HCC | 0.8 | 0.66 | 0.94 | 23 |
| Mortality of compensated cirrhosis | 0.03 | 0.01 | 0.11 | 16 |
| Mortality of compensated cirrhosis with no treated HCC | 0.05 | 0.04 | 0.23 | 3 |
| Mortality of decompensated cirrhosis | 0.21 | 0.15 | 0.27 | 14 |
| Cumulative mortality of decompensated cirrhosis (2 y) | 0.5 | 0.37 | 0.53 | 2 |
| Cumulative mortality of decompensated cirrhosis (3 y) | 0.5 | 0.47 | 0.63 | 1 |
| Cumulative mortality of decompensated cirrhosis (5 y) | 0.7 | 0.6 | 0.8 | 7 |
| Mortality of Early stage of HCC | 0.18 | 0.09 | 0.27 | 5 |
| Mortality of post-surgery resection for HCC (1 y) | 0.15 | 0.12 | 0.21 | 1 |
| Cumulative mortality of post-surgery resection for HCC (3 y) | 0.38 | 0.24 | 0.46 | 1 |
| Mortality of HCC surgery resection for HCC (1 y) | 0.04 | 0.04 | 0.04 | 18 |
| Cumulative mortality of HCC post-surgery resection (5 y) | 0.56 | 0.49 | 0.62 | 4 |
| Mortality of compensated cirrhosis with HCC treated with TACE/PEI | 0.11 | 0.05 | 0.5 | 5 |
| Mortality of decompensated cirrhosis with HCC treated with TACE/PEI | 0.3 | 0.25 | 0.65 | 5 |
| Cumulative mortality of compensated cirrhosis with HCC treated by RFA (5 y) | 0.54 | 0.32 | 0.68 | 2 |
| Cumulative mortality of decompensated cirrhosis with HCC treated by RFA (5 y) | 0.69 | 0.6 | 0.73 | 2 |
| Mortality of liver biopsy | 0.01 | 0.01 | 0.05 | 1 |
| Mortality of liver resection within 30 d | 0.07 | 0.03 | 0.1 | 5 |
| Mortality of liver transplant for decompensated cirrhosis | 0.05 | 0.03 | 0.07 | 16 |
| Mortality of liver transplant surgery for donor | 0.2 | 0.1 | 0.4 | 2 |
| Mortality of post-transplantation | 0.03 | 0.01 | 0.1 | 6 |
| Mortality of radiofrequency ablation | 0 | 0 | 0.02 | 2 |
| Mortality of small HCC | 0.21 | 0.15 | 0.29 | 7 |
| Mortality rate following OLT (1 y)-ALD | 0.08 | 0 | 0.15 | 1 |
| Mortality rate following OLT (1 y)-HBV | 0.22 | 0.1 | 0.34 | 1 |
| Mortality rate following OLT (1 y)-HCV | 0.12 | 0.07 | 0.18 | 1 |
| Cumulative mortality of post-transplantation (5 y) | 0.3 | 0.2 | 0.35 | 1 |
| HCC progression from small to large | 0.33 | 0.16 | 0.5 | 13 |
| HCC progression from small to large after TAE treatment | 0.1 | 0.02 | 0.2 | 3 |
| Progression from compensated cirrhosis to ascites | 0.22 | 0.1 | 0.3 | 2 |
| Progression from compensated cirrhosis to de-compensation | 0.05 | 0.04 | 0.06 | 14 |
| Progression from compensated cirrhosis with HCC surgery resection to de-compensation | 0.1 | 0.01 | 0.25 | 4 |
| HCC progression from small to middle size | 0.3 | 0.2 | 0.4 | 3 |
| Risk of HCC in decompensated cirrhosis | 0.04 | 0.01 | 0.1 | 7 |
| Risk of HCC recurrence | 0.2 | 0.14 | 0.25 | 10 |
| Risk of new HCC | 0.03 | 0.02 | 0.04 | 32 |
| Risk of SBP | 0.18 | 0.06 | 0.29 | 5 |
| Risk of variceal bleeding | 0.17 | 0 | 0.35 | 7 |
Table 2. Summary of model variables for natural history of cirrhosis and HCC used in assessing the cost-effectiveness of HCC screening in cirrhotic patients.
| Variable name | Pooled estimation | Data sources (number of references) | ||
|---|---|---|---|---|
| Mean | 95% CI lower limit | 95% upper limit | ||
| Full health | 1 | 1 | 1 | 2 |
| Compensated cirrhosis | 0.76 | 0.71 | 0.81 | 13 |
| Liver transplantation | 0.71 | 0.64 | 0.78 | 12 |
| Resection and Post resection | 0.67 | 0.52 | 0.83 | 5 |
| Decompensated cirrhosis | 0.64 | 0.57 | 0.72 | 14 |
| No terminal phase HCC | 0.62 | 0.54 | 0.7 | 10 |
| Terminal phase HCC | 0.26 | 0.16 | 0.36 | 11 |
| Death | 0 | 0 | 0 | 8 |
Table 3. Summary of utility variables used in the included studies assessing cost-effectiveness of screening for HCC in cirrhotic patients.
Cost variables by countries
Our study collected cost variables from the Markov models assessing the cost-effectiveness of image-based HCC surveillance in 7 countries (except Thailand). According to the adjusted costs of performing US in 6 countries, US cost the least in the UK (0.002 GDP per capita) and the highest in Italy (0.008 GDP per capita). Of the 4 countries reporting the cost of performing CT, the UK had the least cost (0.005 GDP per capita) and Japan had the highest cost (0.019 GDP per capita). The cost of performing MRI was reported for the UK (0.009 GDP per capita), Switzerland (0.016 GDP per capita), and USA (0.033 GDP per capita).
The included studies also reported medical costs for several main health states in the Markov models. The adjusted annual medical costs for compensated cirrhosis in 5 countries ranged from 0.013 GDP per capita in Japan to 0.061 GDP per capita in Italy. The adjusted annual medical costs for decompensated cirrhosis in 4 countries ranged from 0.137 GDP per capita in Japan to 0.431 GDP per capita in the UK. The included studies from 5 countries reported HCC resection costs that ranged from 0.248 GDP per capita in the UK to 0.666 GDP per capita in the USA. Liver transplantation costs in 3 countries were reported in the included studies and ranged from 1.002 GDP per capita in the UK to 4.456 GDP per capita in the USA. Terminal care costs for advanced HCC were reported in the included studies from 5 countries and ranged from 0.057 GDP per capita in the UK to 0.501 GDP per capita in Switzerland. Other reported cost variables included post-liver transplantation (ranging from 0.377 to 0.608 GDP per capita in the USA, UK, and Italy) and palliative treatment with TACE (ranging from 0.015 to 0.501 GDP per capita in the USA, UK, Switzerland, Japan, and Taiwan).
Base case ICER associated with image-based HCC surveillance versus no surveillance
A summary of baseline cost-effectiveness of screening for HCC in cirrhotic patients in the included studies (ICER per gained LY) is provided in Table 4. Five studies reported base case ICERs per life year associated with HCC surveillance using semi-annual US (0.163 GDP per capita), semi-annual US plus AFP (0.634 GDP per capita), semi-annual CEUS (0.172 GDP per capita), annual US plus AFP (0.543 GDP per capita), and the combination of annual CT and semi-annual AFP (0.603 GDP per capita) for the comparisons with no HCC surveillance in the USA and Japan. In one Japanese study, when using QALY as a health benefit outcome measure, HCC surveillance using semi-annual US was associated with an ICER of 0.930 GDP per capita. The cost-effectiveness of semi-annual US plus AFP was assessed in 3 studies (2 studies from the USA and 1 study from Thailand). The base case ICER per QALY associated with semi-annual US plus AFP was 4.403 GDP per capita but ranged from 0.733 to 1.415 in the two studies from the USA. In one Japanese study, HCC surveillance using semiannual CEUS was cost-effective by having an ICER of 0.382 GDP per capita for additional QALY. Three studies from the USA reported base case ICERs for HCC surveillance using annual US (0.632 GDP per capita), annual US plus AFP (0.599 GDP per capita), semi-annual CT (9.893 GDP per capita), and semi-annual CT plus AFP (0.708 GDP per capita).
| Intervention strategy | Control strategy | ICER (GDP per capital per gained LY) |
|---|---|---|
| US (6-month) | No surveillance | 0.16 |
| CEUS (6-month) | No surveillance | 0.17 |
| US+AFP (12-month) | No surveillance | 0.54 |
| CT+AFP (6-month) | No surveillance | 0.6 |
| AFP+US (6-month) | No surveillance | 0.63 |
| CT (12-month)+AFP (6-month) | US (12-month)+AFP (6-month) | 1.08 |
| AFP+US (6-month) | US (12-month)+AFP (6-month) | 1.76 |
| CT+AFP (12-month) | US+AFP (12-month) | 0.74 |
| CEUS (6-month) | US (6-month) | 0.22 |
| AFP+US (6-month) | CT (12-month)+AFP (6-month) | 2.02 |
| MRI+AFP (6-month) | CT+AFP (6-month) | 2.84 |
| CT+AFP (6-month) | AFP+US (6-month) | 0.41 |
| CT+AFP (6-month) | AFP+US (6-month) | 2.13 |
| US (12-month)+AFP (6-month) | AFP+US (12-month) | 0.74 |
| AFP+US (6-month) | Opportunistic screening | 0.85 |
Table 4. Summary of baseline cost-effectiveness of screening for HCC in cirrhotic patients in the included studies (ICER per gained LY).
Other comparisons
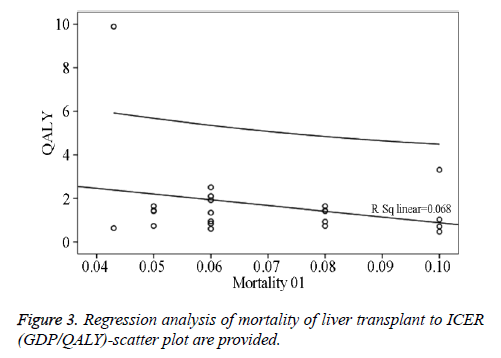
Two studies from the USA compared CT plus AFP versus US plus AFP at 6-month or 12-month surveillance intervals and the reported base case ICERs ranged from 0.466 to 2.514 GDP per capita for additional QALYs and from 0.412 to 2.127 GDP per capita for additional life years. Another 4 studies from the USA, UK, and Italy compared semi-annual AFP plus US versus annual US plus semi-annual AFP, annual CT plus semiannual AFP, annual AFP plus US, and semi-annual AFP, respectively, and the generated base case ICERs per QALY ranged from 1.292 to 2.557 GDP per capita. One study from the USA compared annual US plus semi-annual AFP versus annual US plus AFP and generated a base care ICER of 0.737 GDP per capita for an additional life year or 0.842 GDP per capita for an additional QALY. One Japanese study compared semi-annual ECUS versus semi-annual US and generated a base case ICER of 0.218 GDP per capita for an additional life year or 0.504 GDP per capita for an additional QALY. One study from Taiwan compared semi-annual US plus AFP versus opportunistic screening and reported a base case ICER of 0.850 GDP per capita for an additional QALY. Finally, one study from the USA compared annual MRI plus semi-annual AFP versus annual CT plus semi-annual APF for HCC surveillance and generated a highly unattractive ICER for both an additional life year (2.839 GDP per capita) and QALY (3.312 GDP per capita). Regression analyses of mortality of liver transplant to ICER (GDP/QALY)-scatter plot are provided in Figure 3.
Discussion
Several features of HCC make it a potentially viable target for screening. First, HCC occurs in a well-defined risk population. The primary risk factor is cirrhosis, particularly that related to viral hepatitis. Second, HCC has a protracted subclinical phase. Natural history studies have shown that once cirrhosis is present, up to 20% of patients may develop HCC during the next 10 y [38]. During the subclinical phase, there are often no distinctive symptoms that distinguish patients with HCC from those with only cirrhosis. By the time diagnosis is made, over 85% of tumors are unresectable because of size, multi-focality, hepatic de-compensation, or invasion of the portal vein or surrounding structures [39]. Nonetheless, early detection of this cancer can help doctors to cure it completely [40]. However, for resectable HCCs, the outlook appears to be considerably brighter. Therefore, surveillance is recommended in patients at risk of developing HCC, to identify early-stage tumors that are amenable to curative treatments, thus improving patients’ survival [41,42]. The benefit of HCC surveillance has been demonstrated by a randomized controlled trial performed in chronic HBsAg carriers and confirmed by several cohort studies carried out in cirrhotic patients [43]. Semi-annual surveillance is superior to annual surveillance in terms of cancer stage, amenability to curative/effective treatment and patients’ survival [44]. Nonetheless, in the USA, surveillance for HCC is carried out in less than 20% of at-risk patients, and in Italy, only half of HCCs are diagnosed during surveillance [45].
This study is a systematic review of available evidence about the cost-effectiveness of HCC image-based screening techniques. Similar to a natural river that often finds good paths among many possible paths in its way from the source to its destination; liver cancer screening also comprises many imaging methods [46]. This systematic review included economic evaluation studies conducted in different countries. In general, a screening strategy is likely to be cost- effective in every setting considered and a semi-annual surveillance was shown to be the most cost effective timing strategy. The strategy of surveillance 6-monthly AFP plus US was the most cost effective strategy on all measures, resulting in savings of 4.40 GDP per capita. Compared to no surveillance, this strategy is estimated to more than triple the number of people with operable HCC of less than 3 cm at diagnosis, and almost halve the number of people who die from HCC. However, the cheapest surveillance strategy, annual AFP, which can result in savings of 0.88 GDP still achieved substantial savings compared to no surveillance; for example, it more than doubles the number of operable HCC tumors found, and increases the number of small tumors found more than six-fold. As regards CEUS, a recent study on the cost-effectiveness of surveillance for HCC reported the sensitivity of US at only 28.6% for detecting middle-sized HCC (between 2 and 5 cm in diameter). However, a meta-analysis for the overall sensitivity and specificity of CEUS for the diagnosis of malignant liver lesions was 93% (95% CI: 91-95%) and 90% (95% CI: 88-92%), respectively [47]. The sensitivity of US depends on the skill of the operator, especially in LC patients, in which the intrahepatic echo patterns roughen with advanced fibrosis. When the US sensitivity is expected to be low due to patient physiologic factors, such as obesity, CEUS surveillance is recommended. CEUS sensitivity was a critical factor for costeffectiveness. When the CEUS group was compared to the US group, CEUS surveillance was not cost-effective if its sensitivity was lower than 75%. As noted earlier, CEUS using Sonazoid is effective for Kupffer imaging, and it has been reported to have a high sensitivity [48]. This helps technicians to detect HCC more easily. Thus, CEUS sensitivity greater than 75% represents a reasonable value. On all effectiveness measures (except for the proportion of the cohort who have medium HCCs at diagnosis), surveillance with CT or MRI is as effective as, or slightly more effective than surveillance with US at the same frequency. However, the cost of CT or MRI was higher; its ICER was the largest (9.89 GDP), and its costeffectiveness was the least.
Estimates of the sensitivity and specificity of the imaging instrument, such as US, CEUS, CT, MRI, drawn from screening studies have two shortcomings, from the perspective of a modeling study. First, results are fundamentally tied to the frequency with which surveillance was conducted. Second, the sensitivity of screening tests is almost certainly exaggerated when derived from the surveillance literature.
Discrepancies in the results exist when assessing the type of technology to be used. US in association with AFP technology is likely to be the most cost effective, and the use of CT shows controversial results. Screening should be implemented to detect HCC at an early stage of cirrhosis, and it is likely to be not cost effective as the HCC progresses, or after liver transplantation. Results from Bolondi et al. are likely to be out of the range of the cost effectiveness, which in other studies comparing the same strategies is around $30,000 per QALY [49]. The results are derived from trials and have been conducted by following a societal perspective.
Incidence of HCC recurrence had the strongest association with the cost effectiveness of HCC screening but the association was not statistically significant due to the small number of included studies (regression coefficient 41.899, P=0.357). In general, if the ratio of HCC recurrence increases, the cost will increase, the life year or QALY will decrease, and therefore the ICER will also increase, reducing cost effectiveness. The incidence of HCC is also the key parameter which determines the cost effectiveness of HCC screening (regression coefficient 15.996, P=0.465). The incidence of HCC is the most critical parameter in decision-making for the surveillance of patients with cirrhosis. In our meta-analysis, we calculated a baseline mean value (mean: 0.03, 95% CI 0.02, 0.04), but most studies in Japan reported 5-8% as the incidence of HCC is higher than that in the USA and Europe [50-52].
Mortality of advanced HCC, mortality of compensated cirrhosis, and mortality of liver transplant, which are negatively related to ICER (regression coefficient -33.038, P=0.424), are the key parameters determining the cost effectiveness of HCC screening.
This study used a new method that seems more rational for evaluating the ICER. The differences in type and year of currency make it difficult to compare the cost effectiveness of HCC screening between the included studies. This systematic review adjusted the original ICER reported in the included studies to the current value using the history of inflation rate in the country and then using latest exchange rate to convert the adjusted ICER from the local currency to US$. Because the economic status of a country has a significant impact on the cost effectiveness analysis, this systematic review further adjusted ICER using the latest GDP per capita of the country in order to compare the cost effectiveness of HCC screening strategies across countries.
This system review also has some shortcomings. There was a lack of randomized controlled trials (RCTs) that could help to address many of the questions about the cost effectiveness of HCC screening programs in a real setting. In particular, the organization of healthcare is likely to be the key factor determining the effectiveness and cost-effectiveness of a screening program.
RCTs should be designed following a health technology assessment (HTA)-based approach, considering cost effectiveness as well as organizational, societal, and safety aspects of both the screening techniques and the subsequent treatment. We recognize that an RCT of HCC screening is desirable and represents the ideal study. Further, our results were analyzed based on some assumptions. Thus, although validation is desirable, it is difficult due to ethical issues.
From the results of our analysis, we could indicate that the parameters except the HCC incidence rate, US sensitivity, and CEUS sensitivity have little impact on cost effectiveness. This systematic review also has some limitations, some linked to the search being limited to English, which may have led to the exclusion of some relevant articles. More information is needed as regards which developing technology is the most cost effective. The quality of the studies should be improved and the design of new HTA-based clustered RCTs is needed for the decision-makers to be better supported.
Conclusions
Surveillance for the early detection of HCC is being widely applied around the world. US and serum AFP tests at 6-month intervals are the standard surveillance method. Developing an individualized, highly accurate imaging surveillance strategy based on the risk assessment of a subject will be used to balance cost effectiveness and increase sensitivity in early diagnosis of HCC. We conclude that screening programs for HCC are cost effective when applying US every 6 months to cirrhotic patients for HCC screening. Preventing HCC recurrence could substantially improve the cost effectiveness of image-based screening for HCC in cirrhotic patients.
Acknowledgement
This work was supported by Grant-in-aid for Researchers, Hunan Cancer Hospital, 2013, 5. Zhengping Xiong was sponsored by the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University to complete this research project as his training thesis in the 2013 Canadian Liver Foundation Clinical Research Training Program in Hepatology. Wendong Chen (Division of Social and Administrative Pharmacy, Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, Canada. Health Outcomes Research) and Morris Sherman (Department of Gastroenterology, Toronto General Hospital, University Health Network, Toronto, Canada) participated the discussions on study design and the interpretation of study results.
Author Contributions
Zhengping Xiong formulated the research idea and developed study protocols, design data extraction form, conduct data analyses, and prepare the manuscript draft. The extracted data was validated and the data analyses by Fang Huang. All authors critically revised the manuscript draft.
Conflicts of Interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
References
- Coppola N, Minichini C, Starace M, Sagnelli C, Sagnelli E. Clinical impact of the hepatitis C virus mutations in the era of directly acting antivirals. J Med Virol 2016; 88: 1659-1671.
- Testino G, Leone S, Borro P. Alcoholic liver disease and thehepatitis C virus: an overview and a point of view. Minerva Med 2016; 107: 300-313.
- Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in screening, epidemiology, and end results registries, 1992-2008. Hepatol 2012; 55: 476-482.
- Hocquelet A, Aubé C, Rode A, Cartier V, Sutter O, Manichon AF, Boursier J, N'kontchou G, Merle P, Blanc JF, Trillaud H, Seror O. Comparison of No Touch Multi Bipolar vs. Monopolar radiofrequency ablation for small HCC. J Hepatol 2017; 66: 67-74.
- El-Serag HB, Kramer J, Duan Z, Kanwal F. Epidemiology and outcomes of hepatitis C infection in elderly US Veterans. J Viral Hepat 2016; 23: 687-696.
- Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y, Kudo M, Kubo S, Takayama T, Tateishi R, Fukuda T, Matsui O, Matsuyama Y, Murakami T, Arii S, Okazaki M, Makuuchi M. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res 2015; 45.
- Arslanoglu A, Seyal AR, Sodagari F, Sahin A, Miller FH, Salem R, Yaghmai V. Current Guidelines for the Diagnosis and Management of Hepatocellular Carcinoma: A Comparative Review. AJR Am J Roentgenol 2016; 207: 88-98.
- Kuo MJ, Chen HH, Chen CL, Fann JC, Chen SL, Chiu SY, Lin YM, Liao CS, Chang HC, Lin YS, Yen AM. Cost-effectiveness analysis of population-based screening of hepatocellular carcinoma: Comparing ultrasonography with two-stage screening. World J Gastroenterol 2016; 22: 3460-3470.
- Toy M, Salomon JA, Jiang H, Gui H, Wang H, Wang J, Richardus JH, Xie Q. Population health impact and cost-effectiveness of monitoring inactive chronic hepatitis B and treating eligible patients in Shanghai, China. Hepatol 2014; 60: 46-55.
- Cucchetti A, Cescon M, Erroi V, Pinna AD. Cost-effectiveness of liver cancer screening. Best Pract Res Clin Gastroenterol 2013; 27: 961-972.
- Murakami T, Okada M, Hyodo T. CT versus MR imaging of hepatocellular carcinoma: toward improved treatment decisions. Magn Reson Med Sci 2012; 11: 75-81.
- Giannini EG, Cucchetti A, Erroi V, Garuti F, Odaldi F, Trevisani F. Surveillance for early diagnosis of hepatocellular carcinoma: how best to do it? World J Gastroenterol 2013; 19: 8808-8821.
- Jeong SH, Jang ES, Choi HY, Kim KA, Chung W, Ki M. Current status of hepatitis C virus infection and countermeasures in South Korea. Epidemiol Health 2017; 39: 2017017.
- Andersson KL, Salomon JA, Goldie SJ, Chung RT. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2008; 6: 1418-1424.
- Arguedas MR, Chen VK, Eloubeidi MA, Fallon MB. Screening for hepatocellular carcinoma in patients with hepatitis C cirrhosis: a cost-utility analysis. Am J Gastroenterol 2003; 98: 679-690.
- Goossens N, Singal AG, King LY, Andersson KL, Fuchs BC, Besa C, Taouli B, Chung RT, Hoshida Y. Cost-effectiveness of risk score-stratified hepatocellular carcinoma screening in patients with cirrhosis. Clin Transl Gastroenterol 2017; 8: 101.
- Kaplan DE, Chapko MK, Mehta R, Dai F, Skanderson M, Aytaman A, Baytarian M, D'Addeo K, Fox R, Hunt K, Pocha C, Valderrama A, Taddei TH. Healthcare costs related to treatment of hepatocellular carcinoma among veterans with cirrhosis in the United States. Clin Gastroenterol Hepatol 2017.
- Cucchetti A, Trevisani F, Cescon M, Ercolani G, Farinati F, Poggio PD, Rapaccini G, Nolfo MA, Benvegnù L, Zoli M, Borzio F, Giannini EG, Caturelli E, Chiaramonte M, Pinna AD. Italian Liver Cancer (ITA.LI.CA) Group, Cost-effectiveness of semi-annual surveillance for hepatocellular carcinoma in cirrhotic patients of the Italian Liver Cancer population. J Hepatol 2012; 56: 1089-1096.
- Cadier B, Bulsei J, Nahon P, Seror O, Laurent A, Rosa I, Layese R, Costentin C, Cagnot C, Durand-Zaleski I, Chevreul K; ANRS CO12 CirVir and CHANGH groups. Early detection and curative treatment of hepatocellular carcinoma: A cost-effectiveness analysis in France and in the United States. Hepatol 2017; 65: 1237-1248.
- Kang JY, Lee TP, Yap I, Lun KC. Analysis of cost-effectiveness of different strategies for hepatocellularcarcinoma screening in hepatitis B virus carriers. J Gastroenterol Hepatol 1992; 7: 463-468.
- Khalili M, Guy J, Yu A, Li A, Diamond-Smith N, Stewart S, Chen M Jr, Nguyen T. Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci 2011; 56: 1516-1523.
- Gounder PP, Bulkow LR, Meltzer MI, Bruce MG, Hennessy TW, Snowball M, Spradling PR, Adhikari BB, McMahon BJ. Cost-effectiveness analysis of hepatocellular carcinoma screening by combinations of ultrasound and alpha-fetoprotein among Alaska Native people, 1983-2012. Int J Circumpolar Health 2016; 75: 31115.
- Lin OS, Keeffe EB, Sanders GD, Owens DK. Cost-effectiveness of screening for hepatocellular carcinoma in patients with cirrhosis due to chronic hepatitis C. Aliment Pharmacol Ther 2004; 19: 1159-1172.
- Liu D, Chan AC, Fong DY, Lo CM, Khong PL. Evidence-based surveillance imaging schedule after liver transplantation for hepatocellular carcinoma recurrence. Transplantation 2017; 101: 107-111.
- Nouso K, Tanaka H, Uematsu S, Shiraga K, Okamoto R, Onishi H, Nakamura S, Kobayashi Y, Araki Y, Aoki N, Shiratori Y. Cost-effectiveness of the surveillance program of hepatocellular carcinoma depends on the medical circumstances. J Gastroenterol Hepatol 2008; 23: 437-444.
- Patel D, Terrault NA, Yao FY, Bass NM, Ladabaum U. Cost-effectiveness of hepatocellular carcinoma surveillance in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol 2005; 3: 75-84.
- Vadot L, Boulin M, Guiu B, Aho LS, Vourc'h M, Musat A, Hillon P, Lepage C, Guignard MH, Fagnoni P. Clinical and economic impact of drug eluting beads in transarterial chemoembolization for hepatocellular carcinoma. J Clin Pharm Ther 2015; 40: 83-90.
- Vilgrain V, Abdel-Rehim M, Sibert A, Ronot M, Lebtahi R, Castéra L, Chatellier G. Radio-embolisation with yttrium-90 microspheres versus sorafenib for treatment of advanced hepatocellular carcinoma (SARAH): study protocol for a randomised controlled trial. Trials 2014; 15: 474.
- Cannon RM, Scoggins CR, Callender GG, Quillo A, McMasters KM, Martin RC. Financial comparison of laparoscopic versus open hepatic resection using deviation-based cost modeling. Ann Surg Oncol 2013; 20: 2887-2892.
- Sangiovanni A, Manini MA, Iavarone M, Romeo R, Forzenigo LV, Fraquelli M, Massironi S, Della Corte C, Ronchi G, Rumi MG, Biondetti P, Colombo M. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut 2010; 59: 638-644.
- Sarasin FP, Majno PE, Llovet JM, Bruix J, Mentha G, Hadengue A. Living donor liver transplantation for early hepatocellular carcinoma: A life-expectancy and cost-effectiveness perspective. Hepatol 200; 33: 1073-1079.
- Shih ST, Crowley S, Sheu JC. Cost-effectiveness analysis of a two-stage screening intervention for hepatocellular carcinoma in Taiwan. J Formos Med Assoc 2010; 109: 39-55.
- Sangmala P, Chaikledkaew U, Tanwandee T, Pongchareonsuk P. Economic evaluation and budget impact analysis of the surveillance program for hepatocellular carcinoma in Thai chronic hepatitis B patients. Asian Pac J Cancer Prev 2014; 15: 8993-9004.
- Srisubat A, Chaiwerawattana A, Tanwandee T, Pongchareonsuk P. PCN81 the cost-effectiveness analysis of semi-annual screening for hepatocellular carcinoma in patients with chornic hepatitis B. Value Health 2010; 13: 39.
- Tanaka H, Iijima H, Nouso K, Aoki N, Iwai T, Takashima T, Sakai Y, Aizawa N, Iwata K, Ikeda N, Iwata Y, Enomoto H, Saito M, Imanishi H, Nishiguchi S. Cost-effectiveness analysis on the surveillance for hepatocellular carcinoma in liver cirrhosis patients using contrast-enhanced ultrasonography. Hepatol Res 2012; 42: 376-384.
- Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Jackson S, Ryder S, Cramp M, Stein K. Surveillance of cirrhosis for hepatocellular carcinoma: a cost-utility analysis (Structured abstract). Br J Cancer 2008; 98: 1166-1175.
- McLernon DJ, Dillon J, Donnan PT. Health-state utilities in liver disease: a systematic review. Med Decis Making 2008; 28: 582-592.
- Shirvani-Dastgerdi E, Schwartz RE, Ploss A. Hepatocarcinogenesis associated with hepatitis B, delta and C viruses. Curr Opin Virol 2016; 20: 1-10.
- Khan FS, Ali I, Afridi UK, Ishtiaq M, Mehmood R. Epigenetic mechanisms regulating the development of hepatocellular carcinoma and their promise for therapeutics. Hepatol Int 2016; 11: 45-53.
- Parsian A, Ramezani M, Ghadimi N. A hybrid neural network-gray wolf optimization algorithm for melanoma detection. Biomed Res 2017; 28: 3408-3411.
- Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatol 2005; 42: 1208-1236.
- European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56: 908-943.
- Kolly P, Dufour JF. Surveillance for hepatocellular carcinoma in patients with NASH. Diagnostics (Basel) 2016; 6: 22.
- Santi V, Trevisani F, Gramenzi A, Grignaschi A, Mirici-Cappa F, Del Poggio P, Di Nolfo MA, Benvegnù L, Farinati F, Zoli M, Giannini EG, Borzio F, Caturelli E, Chiaramonte M, Bernardi M. Italian Liver Cancer (ITA.LI.CA) Group, Italian Liver Cancer (ITA.LI.CA) Group. Semi-annual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol 2010; 53: 291-297.
- Singal AG, Yopp A, Skinner CS, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med 2012; 27: 861-867.
- Tarafdar Hagh M, Ebrahimian H, Ghadimi N. Hybrid intelligent water drop bundled wavelet neural network to solve the islanding detection by inverter-based DG. Frontiers in Energy 2015; 9: 75-90.
- Westwood M, Joore M, Grutters J, Redekop K, Armstrong N, Lee K, Gloy V, Raatz H, Misso K, Severens J, Kleijnen J, Kleijnen J. Contrast-enhanced ultrasound using SonoVue® (sulphur hexafluoride microbubbles) compared with contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: a systematic review and cost-effectiveness analysis. Health Technol Assess 2013; 17: 1-243.
- Friedrich-Rust M, Klopffleisch T, Nierhoff J, Herrmann E, Vermehren J, Schneider MD, Zeuzem S, Bojunga J. Contrast-Enhanced Ultrasound for the differentiation of benign and malignant focal liver lesions: a meta-analysis. Liver Int 2013; 33: 739-755.
- Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver 2016; 10: 332-339.
- Wirth TC, Manns MP. The impact of the revolution in hepatitis C treatment on hepatocellular carcinoma. Ann Oncol 2016; 27: 1467-1474.
- Tholey DM, Ahn J. Impact of Hepatitis C Virus Infection on Hepatocellular Carcinoma. Gastroenterol Clin North Am 2015; 44: 761-773.
- de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatol 2015; 62: 1190-1200.