Research Article - Biomedical Research (2017) Volume 28, Issue 8
Cortisol, β-endorphin and oxidative stress markers in healthy medical students in response to examination stress
Kyaimon Myint1*, R Jayakumar2, See-Ziau Hoe1, MS Kanthimathi2,3 and Sau-Kuen Lam1
1Department of Physiology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
2Department of Molecular Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
3University of Malaya Centre for Proteomics Research (UMCPR), University of Malaya, 50603 Kuala Lumpur, Malaysia
- *Corresponding Author:
- Kyaimon Myint
Department of Physiology
Faculty of Medicine, University of Malaya, Malaysia
Accepted date: February 07, 2017
Abstract
Background: The psychosomatic connection pertaining to the relationship between perceived stress and the milieu intérieur that must be evident during a naturalistic stressor event is not well explored.
Objective: This study therefore examines the interrelationship between perceived stress scores, endocrine levels and oxidant-antioxidant activities under the duress of examination stress, an appropriate example of a naturalistic stressor.
Materials and Methods: Apparently healthy year one medical students participated.
Results: Examination stress induced significant increases in perceived stress scores (p<0.001), serum cortisol (p<0.05) and plasma β-endorphin (p<0.05) levels, and erythrocyte lipid peroxidation (p<0.001), but a significant (p<0.001) decrease in the antioxidant superoxide dismutase activity. In addition, during the examination, the perceived stress scores were found to be correlated positively with lipid peroxidation (r2=0.23; p<0.01) but negatively with β-endorphin (r2=0.14; p<0.05). After the examination, the perceived stress scores correlated positively only with cortisol (r2=0.09; p<0.05).
Conclusion: Sitting for an examination increases cortisol secretion, as well as, β-endorphin levels and induces oxidative stress. The high levels of β- endorphin appear to have an ameliorating effect on cortisol and the perception of stress. This finding awaits further investigation.
Keywords
Examination stress, Perceived stress scores, Cortisol, β endorphin, Oxidative stress markers.
Introduction
In daily life, all organisms are faced with various types of dayto- day stresses. Some organisms can cope well with the stress stimuli while in others repeated daily stress could lead to derangement of the neuroendocrine coping mechanism and balance between pro- and anti-oxidants, producing a wide range of detrimental effects on the physiological and psychological homeostasis.
It is well-known that the hypothalamo-pituitary-adrenal (HPA) axis is highly sensitive to and easily activated by various stressors. The axis responds by releasing corticotrophinreleasing hormone (CRH) from the hypothalamus and the consequent adrenocorticotrophic hormone (ACTH) from the anterior pituitary gland. The circulating ACTH then acts on the adrenal cortex causing the release of glucocorticoids (mainly cortisol), the hormonal end-products of the HPAaxis [1], and it is intricately involved in the adaptation to stress.
In the 1970s, beta-endorphin (β-endorphin), a cleavage product of a precursor hormone for ACTH, was discovered by Li and Chung [2]. This compound is an endogenous opiate and is believed to modulate pain, boost the immune system, promote the feeling of wellbeing, and increase relaxation. However, little is known about the role of β-endorphin in acute naturalistic stress [3-6]. Thus, more studies are called for to determine whether endorphins might be released during times of stress that could have significant roles in preventing wear and tear of the body.
Since the pioneering work of Gerschman in the 1950s on oxygen poisoning [7], the role of free radical reactions in humans has been critically reappraised. In the human body, high levels of constantly formed free radicals and other reactive oxygen species (ROS) such as superoxide anion (O2 ?−), hydrogen peroxide (H2O2) and hydroxyl radicals (?OH) are involved in the generation of cascade reactions that attack cell membrane phospholipids and induce membrane lipid peroxidation. In particular, the well-characterised product of lipid peroxidation, malondialdehyde (MDA), can cause damage to proteins and DNA resulting in cellular apoptosis [8]. Hence, protection against and prevention of the consequences of the deleterious effects of ROS are of critical importance and they could possibly be achieved by non-enzymatic (e.g., glutathione, uric acid, bilirubin, vitamin C and E) and enzymatic antioxidants.
Enzymatic defence against ROS-induced tissue damage in humans includes the enzymes superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) [9]. Superoxide dismutase, present in both mitochondria and cytosol, dismutes O2?− to form H2O2 and oxygen (O2) [10], while glutathione peroxidase, found partially within cell membranes, helps in removing H2O2 from body fluids by converting reduced glutathione to oxidized glutathione [11] as well as being able to terminate the chain reaction of lipid peroxidation by removing lipid hydroperoxides from cell membranes [12]. Catalase, located within peroxisomes and in the cytosol of cells, decomposes H2O2 to water and O2. Therefore, increased H2O2 production must be accompanied by increased GPx and/or CAT activity to limit damage. Under normal conditions, a delicate balance between the generation of ROS and heightened antioxidant defence appears to be in place [13]. However, this balance can be readily upset by various factors and this imbalance, the so-called “oxidative stress”, appears to play a pivotal in the pathogenesis of several diseases [13-15].
Stress comes in various forms, and for university students, academic stress poses one of the many challenges that they have to contend with during their university years. A review of the literature reveals a paucity of information regarding this aspect of stress, and in particular, that of the effect of academic stress as a brief naturalist stressor on β-endorphin and oxidative markers mediated through the HPA axis. Thus, this study was undertaken to determine the relationship between perceived stress level, endocrine outcomes and antioxidant activities in medical students undergoing examination stress and their responses thereof.
Materials and Methods
This study was approved by the Medical Ethics Committee of the University Malaya Medical Centre (Approval number: 781.12).
Student recruitment
Announcements about the study were made to the first year medical students at the University of Malaya during their routine tutorial and practical classes. Potential subjects were selected based on their responses to a questionnaire designed to gather information on their past and present medical histories. Only those who reported no histories of acute illness, previous known medical conditions or psychological problems were recruited and they were deemed as normal and healthy participants. Further information about the nature and prerequisites of the investigation were provided to each participant in a face-to-face interview prior to the actual day of experiment, during which written consent and personal history, including socioeconomic status and life-style practices, were also obtained. Participants were reimbursed after each visit.
Experimental design
The study was designed such that the subjective as well as objective parameters for all subjects were obtained during the high-stress period identified as 1 or 2 days before the final written academic examination and during the low-stress period, i.e. within 1 week after the examination.
Subjective assessment: stress questionnaire
Perceived self-rated stress levels were measured by a bilingual; English [16] and Bahasa Malaysia [17] version of the validated 42-item questionnaire developed by Lovibond and Lovibond [16]. It produces sets of scales called the depression anxiety stress scales (DASS) that are widely used in clinical and nonclinical settings to elucidate current state or change in state of mind over time on the three dimensions of depression, anxiety and stress.
In the present study, only the stress scale was used, and the final stress scores obtained from stress scale were derived from the summation of scores for relevant items. The stress scale is constructed to measure the sensitivity levels of chronic and acute nervous arousals that pertain to difficulties in relaxation, feelings of being upset/agitated, irritability, over-reactivity and impatience.
Objective assessment: biochemical analysis
Blood samples were taken from the participants on the same day as the subjective assessment, i.e. between 0800 to 0830 h. The samples were collected in non-heparinised and heparinised tubes and kept in ice until centrifugation. For the nonheparinised tubes, the sera were separated after centrifuging and kept at −80?C until analysed for neurohormones. The blood samples collected in heparinised tubes meant for oxidative stress marker assays were processed as described below.
Neurohormonal assays
Serum cortisol levels were measured by chemiluminescent immunoassays (ADVIA Centaur, USA) while β-endorphin levels were assessed by enzyme immunoassays (Phoenix Pharmaceuticals Inc, USA).
Oxidative stress marker assays
Blood samples from the heparinised tubes were centrifuged at 3000 rpm for 15 minutes, after which plasma from each tube meant for lipid peroxidation was separated out to be followed by removal of the buffy coat layer. The red cell pellet left behind was hemolysed and used for subsequent analyses of antioxidant enzyme activities. The total hemoglobin content was also measured at the same time by the Drabkin method [18].
Lipid peroxidation assay: The plasma MDA activity was determined according to the method of Akku? [19] and used as an index of lipid peroxidation [20,21]. This method is based on the reaction of MDA in plasma with 2-thiobarbituric acid (TBA) at boiling-point temperature that would yield a pink-colour supernatant, the optimal density of which is read spectrophotometrically at 532 and 600 nm, where 532 nm represents the maximum absorbance of the TBA-MDA complex and 600 nm the correction for nonspecific turbidity. The values for absorbance at 600 nm were subtracted from those at 532 nm to give the true MDA values that were expressed as micromoles per gram of hemoglobin (μmol/g Hb).
Antioxidant enzyme activity: Determination of antioxidant status was obtained by the activities of SOD, CAT and GPx that were estimated from the erythrocyte haemolysates. Total SOD activity was determined by the method of Kakkar [22] in which reduction of the substrate, nitroblue tetrazolium (NBT), is used to indicate O2?− production. One unit (U) of the SOD activity inhibits the rate of reduction of NBT by 50%. Catalase activity was measured by measuring the breakdown of H2O2 at 240 nm [23]. One unit of CAT decomposes one μmole of H2O2 per min with CAT activity being expressed as U/g Hb. The GPx activity was assessed by the oxidation of glutathione by H2O2. This reaction is coupled to the reduction of oxidized glutathione by glutathione reductase, which simultaneously oxidizes NADPH to NADP+ [24]. The decrease in absorbance at 340 nm is expressed as units per gram of hemoglobin (U/g Hb).
Statistical analysis
All parameters are reported as means ± SEM. A paired sample t-test was used to compare the scores of the subjective as well as objective parameters during the stress and non-stress periods. Pearson correlations were performed at each time period to ascertain possible associations among all parameters. A probability value of less than 0.05 (p<0.05) is taken to be significant.
Results
The participants
Thirty-three students,cmale (n=19) and female (n=14) successfully completed the study. The participants were aged 20.0 ± 0.08 y, single, did not smoke or drink, and were free from medication and financial constraints. They also have no history of previously known medical conditions or psychological problems. No significant difference in these personal data between males and females was found.
Perceived stress scores, cortisol and β-endorphin and oxidative stress markers
The perceived stress scores, serum cortisol and β-endorphin levels and MDA, SOD, CAT, GPx activities during the highstress and low-stress periods are shown in Table 1. During the high-stress period, the participants showed significantly higher levels of perceived stress (p<0.001), serum cortisol (p<0.05) and β-endorphin (p<0.05) compared with the low-stress period. In addition, the MDA activity, a measure of lipid peroxidation, was significantly (p<0.001) reduced upon completion of the examination, while a marked (p<0.001) increase in the antioxidant SOD activity was observed at the same time. The CAT and GPx activities remained unchanged during low-stress period.
| Parameters | High-stress period | Low-stress period | p value |
|---|---|---|---|
| Perceived Stress levels | |||
| DASS Stress scores | 12.48 ± 0.77 | 7.27 ± 1.13 | *** |
| Neurohormone levels | |||
| Serum cortisol (nmol/L) | 465.45 ± 16.29 | 427.73 ± 18.64 | * |
| Serum β-endorphin (pg/ml) | 148.74 ± 22.03 | 78.52 ± 38.16 | * |
| Lipid Peroxidation | |||
| MDA activity (mmol/g Hb) | 0.31 ± 0.01 | 0.23 ± 0.00 | *** |
| Antioxidant activities | |||
| SOD activity (U/g Hb) | 1572.93 ± 26.74 | 1799.70 ± 24.65 | *** |
| CAT activity (U/g Hb) | 3728.12 ± 94.23 | 3790.11 ± 67.62 | NS |
| GPx activity (U/g Hb) | 24.24 ± 0.68 | 25.84 ± 0.56 | NS |
*p<0.05, ** p<0.01, *** p<0.001 and NS: Not significant, p>0.05 versus low-stress period
Table 1: The perceived stress scores, cortisol and β-endorphin levels and oxidative stress marker activities (mean ± SEM) during high- and low-stress period (n=33).
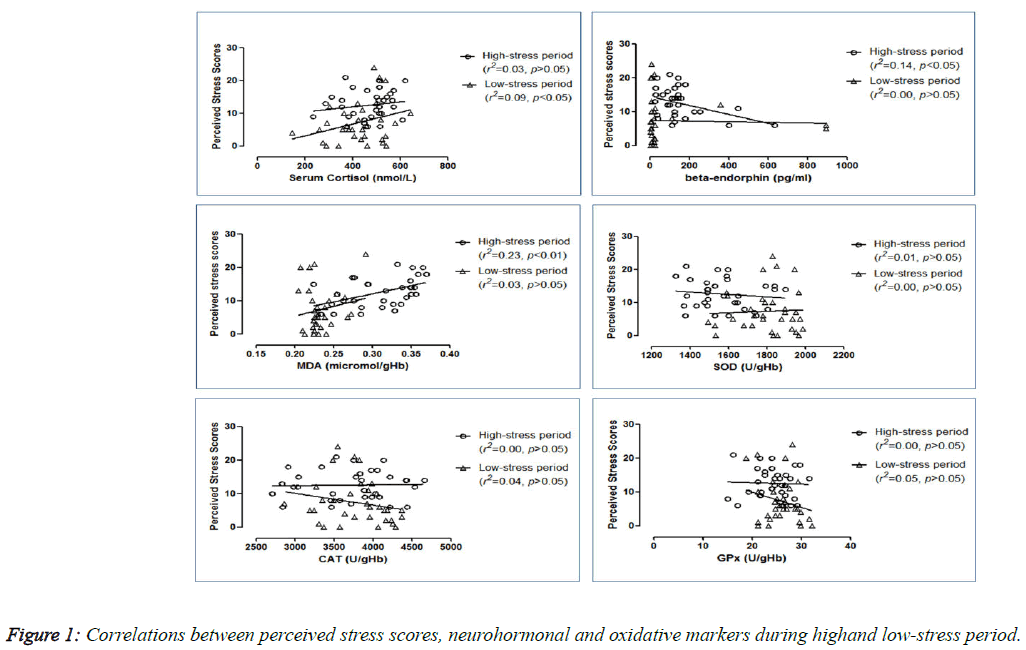
Correlations between perceived stress scores and blood parameters
The correlations between the perceived stress scores and other parameters at both periods are shown in Figure 1. During the high-stress period, perceived stress scores were found to be correlated directly to MDA (r2=0.23; p<0.01) but inversely to β-endorphin (r2=0.14; p<0.05). With low-stress, only cortisol demonstrated direct correlation with the perceived stress scores (r2=0.09; p<0.05). However, no significant correlations were found for β-endorphin (r2=0.00; p>0.05) and MDA (r2=0.03; p>0.05) during that period. In addition, no significant correlations were found between perceived stress scores and the anti-oxidative enzymes in the high-stress period (activities of SOD (r2=0.01; p>0.05), CAT (r2=0.00; p>0.05) and GPx (r2=0.00; p>0.05) as well as in the low-stressed period (SOD (r2=0.00; p>0.05), CAT (r2=0.04; p>0.05) and GPx (r2=0.05; p>0.05) activities) (graphs not shown).
Discussion
This investigation confirms and extends previous work that clearly demonstrates the effect of examination stress on stress hormones. Taking academic examination as a brief naturalistic stressor, there is evidence that significantly high levels of perceived stress, serum cortisol and β-endorphin have been evoked. Furthermore, convincing evidence of an involvement of the pro- and anti-oxidation system in the physiological response to stress as well as a novel finding of a role played by β-endorphin are also put forward. It was previously shown that both the nature of the stressor and the state of the respondent are important in determining the endocrine responses to stress. Therefore, in this study, a DASS was used to assess the perceived stress levels of participants as it has been soundly validated in the literature [16,17] as a reliable tool to sensitively reflect the changes in mood, anxiety and distress in individuals or groups.
Although there are substantial researches done on examination stress and endocrine changes, the results showed inconsistent findings [3,25-30]. Moreover, the studies of cortisol levels by the cortisol awakening response, a new indicator of individual’s HPA axis activation, also showed the inconsistent responses of cortisol with the experience of stress itself [31-33]. In this study, significantly high perceived stress levels (p<0.001) before the examination indicated that academic examinations are indeed powerful inducers of perceived stress in students. Although serum cortisol levels were significantly increased (p<0.05) during the high-stress period, it was not correlated with perceived stress scores suggesting the importance of individual differences in determining the endocrine profile to stress [26]. During the low-stress period, perceived stress scores were found to be positively correlated with cortisol (p<0.05). Thus, this clearly demonstrated a heightened HPA involvement during the examination period (under stress) which was quickly and significantly resolved once the examination was over. These data may therefore help to elucidate more definitively the positive response of cortisol to acute stress [3,25,26,30,31,33]. However, there had been few reports of no change and even reductions in cortisol secretion have also occasionally surfaced [27-29,32].
The involvement of the so-called happy hormone, β-endorphin, in acute stress has not been well studied in the literature and may play an important role in the response of the HPA axis and release of cortisol under the duress of stress. In this study, β- endorphin levels were increased (p<0.05) during high-stress period when compared to low-stress period, and it was negatively correlated (r2=0.14; p<0.05) with perceived stress scores. However, no correlation was found between the two hormones. The particular interplay between cortisol and β- endorphin can be explained by the notion that stress itself has a profound effect on the HPA axis wherein stress per se stimulates the hypothalamic release of CRH, which appears to induce the expression of the proopiomelanocortin (POMC) gene in the anterior pituitary, causing a concurrent secretion of ACTH and β-endorphin from anterior pituitary [1]. It is speculated that the rise in β-endorphin would then attenuate the secretion of cortisol, possibly due partly to the negative feedback of β-endorphin on the HPA itself [34,35], resulting in a lower than expected level of cortisol during the high-stress period. Indeed, such is the power of the benefits of β- endorphin that even the stress scores obtained during the highstress period appear to be lower, giving rise to the its inverse correlation with perceived stress scores seen in Figure 1. Furthermore, during the high-stress period, due to the lower than expected cortisol levels, a positive correlation with the stress scores could not be discerned. However, during the lowstress period, a significant positive correlation between stress scores and the lingering cortisol levels was obtained (Figure 1). This could be due to the diminishing ameliorating influence of β-endorphin during low-stress period. Thus, it is feasible to suggest that this increase in β-endorphin could provide a central mechanism for the individual to cope with the stress evoked, and may perhaps also help temper the vulnerabilities of individuals to stress-related psychopathology.
To determine the oxidative stress markers, we used red blood cells as they have high levels of SOD, CAT and GPX activities. Sitting for an academic examination can significantly increase the pro-oxidant MDA while decreasing the anti-oxidant SOD levels. These findings are supported by the positive correlation found between stress and MDA (Figure 1), and are similar to those reported by others [36-38]. As SOD is the first enzyme involved in the metabolism or destruction of superoxide anion radicals, the decreased activity of SOD would cause the formation of high levels of ROS, and subsequently, increase in membrane lipid peroxidation. Although not significant, the other two antioxidant markers, CAT and GPx, showed lower levels during the examination period, and these low levels might contribute the accumulation of H2O2 in the body. Thus, examination stress apparently shifts the delicate pro- and antioxidation balance to a more pro-oxidative state. While a single episode of exposure to brief naturalistic stressor may not predispose an individual to the damaging effects of elevated ROS, it is possible that repeated activation may subject the susceptible individual to increased allostatic load and risk of chronic ROS-related diseases [10,13,14]. This may also lend support to the anecdotal practice of some individuals consuming antioxidant-rich supplementation during an examination period.
This study has limitations. First, single samples of cortisol level were measured in this study. Although a single morning measurements of cortisol have been reported to be a reliable measure of HPA axis activity, the measures of cortisol secretion with strict reference to the time of awakening [39,40] may have revealed additional results. In addition, as the sample size was small and limited to first year students, these findings may not be generalized to the other year groups of medical students. Further studies are needed to be carried out to address these limitations.
Conclusion
The results of this study confirm and strengthen the hypothesis that brief naturalistic stressors such as an academic examination stimulate both the HPA axis and the endogenous opiate system. Even though cortisol and β-endorphin works independently, the stress-induced release of β-endorphin attenuates the HPA axis and hence cortisol secretion which, in turn, may contribute to lessening of the adverse effects of stress. Sitting for an academic examination shifts the pro- and anti-oxidation balance to a more pro-oxidative state through the impairment of lipid oxidation and enzymatic antioxidant defences. Further studies on the detailed mechanism underlying β-endorphin release and oxidative markers may yield insights into the role of these substances in the allostasis of the stress response.
Acknowledgements
This study is funded by grants (RG266/10HTM and RG341/11HTM) from the University Malaya Research Grant Health and Translational Medicine Cluster. We wish to thank Dr Khang Tsung Fei, senior lecturer, from Faculty of Science, University of Malaya for his expertise and advice on the statistical analyses. We would also like to thank the medical students who participated in this study.
References
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol 2005; 67: 259-284.
- Li CH, Chung D. Isolation and structure of an untriakontapeptide with opiate activity from camel pituitary glands. Proc Natl Acad Sci 1976; 73: 1145-1148.
- Malarkey WB, Pearl DK, Demers LM, Kiecolt-Glaser JK, Glaser R. Influence of academic stress and season on 24-hour mean concentrations of ACTH, cortisol, and ß-endorphin. Psychoneuroendocrinol 1995; 20: 499-508.
- Stanojevic S, Mitic K, Vujic V, Kovacevic-Jovanovic V, Dimitrijevic M. Exposure to acute physical and psychological stress alters the response of rat macrophages to corticosterone, neuropeptide Y and beta-endorphin. Stress 2007; 10: 65-73.
- Kavushansky A, Kritman M, Maroun M, Klein E, Richter-Levin G. ß-endorphin degradation and the individual reactivity to traumatic stress. Eur Neuropsychopharmacol 2013; 23: 1779-1788.
- Savic D, Knezevic G, Matic G, Damjanovic S, Spiric Z. Posttraumatic and depressive symptoms in ß-endorphin dynamics. J Affect Disord 2015; 181: 61-66.
- Gerschman R, Gilbert DL, Nye SW, Dwyer P, Fenn WO. Oxygen poisoning and x-irradiation: a mechanism in common. Science 1954; 119: 623-626.
- Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysiology 2000; 7: 153-163.
- Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev 1994; 74: 139-162.
- McCord JM, Edeas MA. SOD, oxidative stress and human pathologies: a brief history and a future vision. Biomed Pharmacother 2005; 59: 139-142.
- Halliwell B, Foyer CH. Properties and physiological function of a glutathione reductase purified from spinach leaves by affinity chromatography. Planta 1978; 139: 9-17.
- McCray PB, Gibson DD, Fong KL, Hornbrook KR. Effect of glutathione peroxidase activity on lipid peroxidation in biological membranes. Biochim Biophys Acta 1976; 431: 459-468.
- McCord JM. Human disease, free radicals, and the oxidant/antioxidant balance. Clin Biochem 1993; 26: 351-357.
- Aruoma OI. Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc 1998; 75: 199-212.
- Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol 2015; 4: 180-183.
- Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. 2nd ed Sydney: School of Psychology, University of New South Wales 2004.
- Mazalisah M, Rusli BN, Naing L, Edimansyah B. Validation of the Malay version of the Depression Anxiety Stress Scales 21-item in an automobile industry. Malays J Med Sci 2005; 12: 250.
- Drabkin D, Austin H. Spectrophotometric studies: II. Preparations from washed blood cells; nitric oxide hemoglobin and sulfhemoglobin. J Biol Chem 1935; 112: 51-65.
- Akkus I, Saglam NI, Çaglayan O, Vural H, Kalak S, Saglam M. Investigation of erythrocyte membrane lipid peroxidation and antioxidant defense systems of patients with coronary artery disease (CAD) documented by angiography. Clinica Chimica Acta 1996; 244: 173-180.
- Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 1990; 186: 421-431.
- Lazzarino G, Tavazzi B, Di Pierro D, Vagnozzi R, Penco M, Giardina B. The relevance of malondialdehyde as a biochemical index of lipid peroxidation of postischaemic tissues in the rat and human beings. Biol Trace Elem Res 1995; 47: 165-170.
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 1984; 21: 130-132.
- Sinha AK. Colorimetric assay of catalase. Anal Biochem 1972; 47: 389-394.
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG. Selenium: biochemical role as a component of glutathione peroxidase. Science 1973; 179: 588-590.
- Al-Ayadhi LY. Neurohormonal changes in medical students during academic stress. Ann Saudi Med 2005; 25: 36-40.
- Weekes N, Lewis R, Patel F, Garrison-Jakel J, Berger DE, Lupin SJ. Examination stress as an ecological inducer of cortisol and psychological responses to stress in undergraduate students. Stress 2006; 9: 199-206.
- Loft P, Thomas MG, Petrie KJ, Booth RJ, Miles J, Vedhara K. Examination stress results in altered cardiovascular responses to acute challenge and lower cortisol. Psychoneuroendocrinol 2007; 32: 367-375.
- Takatsuji K, Sugimoto Y, Ishizaki S, Ozaki Y, Matsuyama E, Yamaguchi Y. The effects of examination stress on salivary cortisol, immunoglobulin A, and chromogranin A in nursing students. Biomed Res 2008; 29: 221-224.
- Myint K, Choy KL, Su TT, Lam SK. The effect of short-term practice of mindfulness meditation in alleviating stress in university students. Biomed Res 2011; 22: 165-171.
- Maduka IC, Neboh EE, Ufelle SA. The relationship between serum cortisol, adrenaline, blood glucose and lipid profile of undergraduate students under examination stress. Afr Health Sci 2015; 15: 131-136.
- Hewig J, Schlotz W, Gerhards F, Breitenstein C, Lürken A, Naumann E. Associations of the cortisol awakening response (CAR) with cortical activation asymmetry during the course of an exam stress period. Psychoneuroendocrinol 2008; 33: 83-91.
- Duan H, Yuan Y, Zhang L, Qin S, Zhang K, Buchanan TW, Wu J. Chronic stress exposure decreases the cortisol awakening response in healthy young men. Stress 2013; 16: 630-637.
- Gonzalez-Cabrera J, Fernandez-Prada M, Iribar-Ibabe C, Peinado JM. Acute and chronic stress increase salivary cortisol: a study in the real-life setting of a national examination undertaken by medical graduates. Stress 2014; 17: 149-156.
- Bilkei-Gorzo A, Racz I, Michel K, Mauer D, Zimmer A, Klingmüller D, Zimmer A. Control of hormonal stress reactivity by the endogenous opioid system. Psychoneuroendocrinol 2008; 33: 425-436.
- Kobayashi Y, Mizusawa K, Arai Y, Chiba H, Takahashi A. Inhibitory effects of ß-endorphin on cortisol release from goldfish (Carassius auratus) head kidney: an in vitro study. Gen Comp Endocrinol 2014; 204:126-134.
- Sivonová M, Zitnanová I, Hlincíková L, Skodácek I, Trebatická J. Oxidative stress in university students during examinations. Stress 2004; 7: 183-188.
- Eskiocak S, Gozen AS, Yapar SB, Tavas F, Kilic AS, Eskiocak M. Glutathione and free sulphydryl content of seminal plasma in healthy medical students during and after exam stress. Hum Reprod 2005; 20: 2595-2600.
- Rostami A, Boojar MM, Adibi P, Changiz T. Level of oxidative stress markers among physicians in a medical residency program. Arch Environ Occup Health 2008; 63: 154-158.
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med 1999; 61: 197-204.
- Schulz P, Kirschbaum C, Prüssner J, Hellhammer DH. Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Med 1998; 14: 91-97.