Research Article - Biomedical Research (2017) Volume 28, Issue 2
Correlations of zinc-fingers and homeoboxes 2 (ZHX2), p53, and survivin expression in a variety of malignant tumors
Zili Lv*, Yangjun Du, Weijia Mo and Guoyu ZhaoDepartment of Pathology, the First Affiliated Hospital of Guangxi Medical University, Nanning 530021, China
- *Corresponding Author:
- Zili Lv
Department of Pathology
The First Affiliated Hospital of Guangxi Medical University
Nanning 530021, China
Accepted date: July 25, 2016
Abstract
This study aims to investigate the expression and correlations of the transcriptional repressors zincfingers and homeoboxes 2 (ZHX2), p53, and survivin in various malignant tumors. Immunohistochemistry was performed to detect the expression of ZHX2, p53, and survivin in the tumor tissues and corresponding paraneoplastic normal tissues of 36 cases of esophageal cancer, 38 cases of gastric cancer, 42 cases of lung cancer, and 38 cases of breast cancer. Their mutual correlations and relationships with clinicopathological features were also analyzed. The expression of ZHX2 in the above cancer tissues was higher than that in the corresponding paraneoplastic normal tissues, with statistically significant differences (P<0.05). In esophageal cancer, the expression of ZHX2 was related to the invasion depth of the tumor, while not being associated with the expressions of p53 and survivin. In gastric cancer the expression of ZHX2 was also related to the invasion depth of the tumor, but was positively correlated with the expression of p53 (P=0.02, r=0.376) and survivin (P=0.015, r=0.391). For lung cancer, the expression of ZHX2 was correlated with the presence or absence of mediastinal lymph node metastasis (P <0.05), and positively correlated with the expression of p53 (P=0.000, r=0.513). Finally, in the breast cancer samples, the expression of ZHX2 was related to the presence or absence of axillary lymph node metastasis (P<0.05), whilst not being linked to the expression of estrogen and prostaglandin. Therefore, the expression of ZHX2 correlated with the occurrence, development, and prognosis judgment of a variety of malignant tumors.
Keywords
Transcriptional repressor ZHX2, Esophageal cancer, Gastric cancer, Lung cancer, Breast cancer.
Introduction
Malignant tumors are one of the greatest threats to human health, and their occurrence and development are governed by multiple genes and their multi-step actions. Many oncogenes [1], tumor suppressor genes [2], and cell cycle-associated genes [3] are involved in carcinoma occurrence and development. Zinc-fingers and homeoboxes 2 (ZHX2) is a transcriptional repressor and a member of the human ZHX family that also includes ZHX1 and ZHX3. It can form homodimers and regulate a number of nuclear factor-YA (NFYA)- regulated genes via an organized transcription network [4]. A recent study showed that ZHX2 represses expression of alpha-fetoprotein (AFP) [5], glypican-3 (GPC3) [6], and H19 [7], and reduces multidrug resistance protein 1 (MDR1) [4] expression in liver cancer cells. ZHX2 abnormal expression is related with tumorigenesis of hepatocellular carcinoma (HCC) [5-7], myeloma [8], and lymphoma [9], but, until now, there have been no reports on ZHX2 protein expression in lung, esophageal, and breast carcinomas. p53 is a human tumor suppressor gene, and imbalance in its mutation and expression could lead to the occurrence of a variety of tumors [10]. Survivin is a new member of the inhibitor of apoptosis (IAp) family and is currently the most powerful inhibitor of apoptosis found. It is highly expressed in a variety of tumors [11]. How does ZHX2 work in the occurrence and development of these and other cancers? Does ZHX2 interact with p53 and survivin? The answers to these questions are unknown. In this study, we investigated the expressions of ZHX2, p53, and survivin and their relationships in many common human carcinomas.
Materials and Methods
Tissues and patients
The tumor tissues and corresponding paraneoplastic normal tissues of 36 cases of esophageal cancer (squamous cell carcinoma), 38 cases of gastric cancer (adenocarcinoma), 42 cases of lung cancer (including 12 cases of squamous cell carcinoma, 19 cases of adenocarcinoma, five cases of glandular squamous cell carcinoma, and six cases of neuroendocrine carcinoma), and 38 cases of breast cancer (invasive ductal cancer) were collected from the Department of Pathology, the First Affiliated Hospital of Guangxi Medical University, from January 2010 to January 2014. The pathological grading referred to the tumor classification and diagnostic criteria of WHO, and the tumor size, invasion depth, and staging criteria referred to the tumor lymph node metastasis (TNM) staging systems of the above tumors developed by the Union for International Cancer Control (UICC); all the patients did not undergo preoperative radiotherapy, chemotherapy, or immunotherapy. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Guangxi Medical University. Written informed consent was obtained from all participants.
Immunohistochemistry
The dewaxing of paraffin sections and EDTA high-pressure hot antigen retrieval was performed. The sections were then incubated with 3% H2O2 at 37°C for 10 min to remove the activities of endogenous peroxidase. The primary antibodies of ZHX2 (diluted 1:2000, Santa Cruz Biotechnology Co., Dallas, USA), p53(DAKO, North America, Inc., Carpinteria, CA, USA), and survivin (DAKO, North America, Inc.) were added in a drop-wise manner and incubated at 37°C for 1.5 h, and rinsed with PBS. The secondary antibodies were added drop by drop and the incubation continued at 37°C for 30 min before being rinsed with PBS. Following this, 3,3'-diaminobenzidine (DAB; DAKO, North America, Inc.) coloring, hematoxylin restaining, dehydration, hyalinization, and mounting with neutral gum was performed. The negative control used PBS to replace the primary antibodies.
Results judgment
The expressions of ZHX2 could be classified into four levels based on the staining depth of positive cells: unstained (0 points), light yellow (1 point), buff (2 points), and tan (3 points). Based on the proportions of positive cells, the expressions of ZHX2 could be divided into five levels: <10% (0 points), 10-25% (1 point), 26-50% (2 points), 51-75% (3 points), and >75% (4 points). The sum of these two scores was then divided into two levels: 0-3 points, to give negative (0) and weakly positive (1+) groups; and 4-7 points, for moderately (2+) and strongly positive (3+) groups. p53 and survivin results were divided into positive and negative, according to the proportion of positive cells, with >10% considered as positive [12].
Statistical analysis
SPSS16.0 statistical software was used to analyze the expressions of ZHX2, p53, and survivin in the tumors and adjacent tissues, as well as the relationships among these three factors and with the clinical pathologies (χ2 test or Fisher's exact test); to determine the correlations among ZHX2, p53, and survivin, the Spearman’s rank correlation analysis was performed, with test level set as α=0.05.
Results
Expression of ZHX2
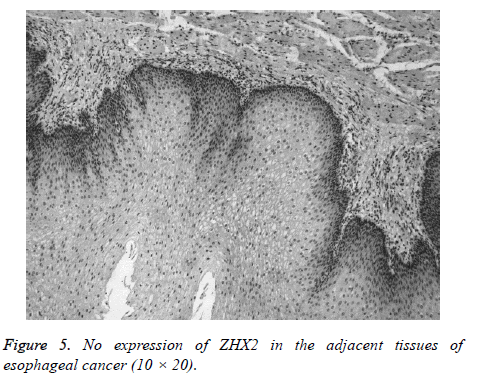
ZHX2 protein was detected in the nuclei and the expression was positive in carcinoma tissues. The stain was brown or tan (Figures 1-4) and the expression was negative (0) or weakly positively (1+) in adjacent normal tissues (Figure 5). Among the 36 cases of esophageal carcinoma, 30 cases scored 2+ and 3+, and six cases of adjacent tissue samples scored 2+ and 3+ (P=0.000). Of the 38, 20 cases of the gastric cancer and five cases of adjacent tissue samples scored 2+ and 3+ (P=0.000). There were 33 cases that scored 2+ and 3+ among the 42 cases of lung cancer, and 15 cases of adjacent tissue samples also scoring 2+ and 3+ (X2=50.524, P=0.000). Again, scores of 2+ and 3+ were observed for 28 cases of breast cancer, of the 38, and the 12 cases of adjacent tissue samples. The positive expression rates of ZHX2 in the above various cancer tissues were significantly higher than those in the adjacent tissues (Table 1).
| Tumor | (0)-(1+) | (2+)-(3+) | X2 | P |
|---|---|---|---|---|
| Esophageal cancer | 6 | 30 | / | 0 |
| Adjacent tissues of esophageal cancer | 30 | 6 | ||
| Gastric cancer | 18 | 20 | / | 0 |
| Adjacent tissue of Gastric cancer | 33 | 5 | ||
| Lung Cancer | 9 | 33 | 15.75 | 0 |
| Adjacent tissues of lung cancer | 27 | 15 | ||
| Breast cancer | 10 | 28 | / | 0 |
| Adjacent tissues of breast cancer | 26 | 12 |
Table 1. Expressions of ZHX2 in different tumor tissues and adjacent normal tissues.
Expressions of p53
p53 expression was positively detected mainly in carcinoma cells, but was predominantly negative in adjacent tissues. Among the 36 cases of esophageal carcinoma, 21 cases were positive; nine cases of adjacent tissue samples were also positive (P=0.000). For the 38 cases of gastric cancer, 21 cases were positive; three cases of adjacent tissue samples were also positive (P=0.000). Twelve of the 42 lung cancer cases were positive, with one case of adjacent tissue samples also being positive (X2=11.012, P=0.000). From the 38 cases of breast cancer investigated, it was observed that 13 cases were positive, but no case of adjacent tissue samples was positive (P=0.000) (Table 2).
| Tumor | - | + | X2 | P |
|---|---|---|---|---|
| Esophageal cancer | 15 | 21 | / | 0 |
| Adjacent tissues of esophageal cancer | 27 | 9 | ||
| Gastric cancer | 17 | 21 | / | 0 |
| Adjacent tissue of Gastric cancer | 35 | 3 | ||
| Lung Cancer | 30 | 12 | 11.012 | 0.001 |
| Adjacent tissues of lung cancer | 41 | 1 | ||
| Breast cancer | 25 | 13 | / | 0 |
| Adjacent tissues of breast cancer | 38 | 0 |
Table 2. Expressions of p53 in different tumor tissues and adjacent normal tissues.
Expression of survivin
Survivin was positively detected in the cytoplasm and/or nuclei of mainly carcinoma cells, but was not detected in adjacent tissue. Among the 36, 31 cases of esophageal carcinoma and seven cases of adjacent tissue samples were positive (P=0.000). Of the 38 cases of gastric cancer, there were 28 positive cases. Six cases of the adjacent tissue samples were also positive (P=0.000). There were 24 positive cases from the 42 cases of lung cancer, with four cases of adjacent tissue samples also being positive (X2=21.429, P=0.000). Of the 38 cases of breast cancer, there were 31 cases that were positive, with further seven positive cases found in the adjacent tissue samples (P=0.000) (Table 3).
| Tumor | - | + | X2 | P |
|---|---|---|---|---|
| Esophageal cancer | 5 | 31 | / | 0 |
| Adjacent tissues of esophageal cancer | 29 | 7 | ||
| Gastric cancer | 10 | 28 | / | 0 |
| Adjacent tissue of Gastric cancer | 32 | 6 | ||
| Lung Cancer | 18 | 24 | 21.429 | 0 |
| Adjacent tissues of lung cancer | 38 | 4 | ||
| Breast cancer | 7 | 31 | / | 0 |
| Adjacent tissues of breast cancer | 31 | 7 |
Table 3. Expressions of survivin in different tumor tissues and adjacent normal tissues.
Relationships of ZHX2 and clinicopathological features
In esophageal cancer, the expression of ZHX2 was related to the invasion depth (P=0.035) whereas in gastric cancer, the expression of ZHX2 was related to the tumor size and invasion depth (P=0.001 and P=0.032, respectively). Investigations into lung cancer showed the expression of ZHX2 was associated with age, tumor size, differentiation degree, and lymph node metastasis (P=0.046, 0.010, 0.017, and 0.029, respectively). Likewise, in breast cancer the expression of ZHX2 was linked with age, tumor size, differentiation degree, and lymph node metastasis (P=0.043, 0.016, 0.016, and 0.018, respectively) (Tables 4-7).
| Group | ZHX2 | P | |
|---|---|---|---|
| (0)-(1+) | (2+)-(3+) | ||
| Gender | |||
| M | 5 | 19 | 0.331 |
| F | 1 | 11 | |
| Age | |||
| <60 years | 3 | 16 | 0.614 |
| ≥ 60 years | 3 | 14 | |
| Grade | |||
| 1~2 | 3 | 20 | 0.645 |
| 3~4 | 3 | 10 | |
| Invasion | |||
| T1~T2 | 5 | 10 | 0.035 |
| T3~T4 | 1 | 20 | |
| LN metastasis | |||
| No | 4 | 17 | 0.507 |
| Yes | 2 | 13 | |
Table 4. Relationships of ZHX2 and clinicopathological features in esophageal cancer.
| Group | ZHX2 | P | |
|---|---|---|---|
| (0)-(1+) | (2+)-(3+) | ||
| Gender | |||
| M | 14 | 12 | 0.205 |
| F | 4 | 8 | |
| Age | |||
| <60 years | 10 | 12 | 0.52 |
| ≥ 60 years | 8 | 8 | |
| Size | |||
| <5 cm | 12 | 3 | 0.001 |
| ≥ 5 cm | 6 | 17 | |
| Grade | |||
| 1~2 | 1 | 3 | 0.344 |
| 3~4 | 17 | 17 | |
| Invasion | |||
| T1~T2 | 6 | 1 | 0.032 |
| T3~T4 | 12 | 19 | |
| LN metastasis | |||
| No | 3 | 4 | 0.563 |
| Yes | 15 | 16 | |
Table 5. Relationships of ZHX2 and clinicopathological features in gastric cancer.
| Group | ZHX2 | X2 | P | |
|---|---|---|---|---|
| (0)-(1+) | (2+)-(3+) | |||
| Gender | ||||
| M | 7 | 19 | 1.224 | 0.269 |
| F | 2 | 14 | ||
| Age | ||||
| <60 | 3 | 23 | 3.965 | 0.046 |
| ≥ 60 | 6 | 10 | ||
| Size | ||||
| <5 cm | 7 | 10 | 6.615 | 0.01 |
| ≥ 5 cm | 2 | 23 | ||
| Grade | ||||
| 1~2 | 7 | 11 | 5.704 | 0.017 |
| 3~4 | 2 | 22 | ||
| Type | ||||
| Squamous cell carcinoma | 3 | 9 | 0.129 | 0.981 |
| Adenocarcinoma | 4 | 15 | ||
| Adeno-squamous cell carcinoma | 1 | 4 | ||
| N endocrine | 1 | 5 | ||
| LN metastasis | ||||
| No | 6 | 9 | 4.78 | 0.029 |
| Yes | 3 | 24 | ||
Table 6. Relationships of ZHX2 and clinicopathological features in lung cancer.
| Group | ZHX2 | P | |
|---|---|---|---|
| (0)-(1+) | (2+)-(3+) | ||
| Age | |||
| <50 | 2 | 18 | 0.043 |
| ≥ 50 | 7 | 11 | |
| Size | |||
| <5 cm | 6 | 6 | 0.016 |
| ≥ 5 cm | 3 | 23 | |
| Grade | |||
| 1~2 | 6 | 6 | 0.016 |
| 3~4 | 3 | 23 | |
| LN metastasis | |||
| No | 7 | 9 | 0.018 |
| Yes | 2 | 20 | |
| ER | |||
| (-) | 4 | 15 | 0.5 |
| (+) | 5 | 14 | |
| PR | |||
| (-) | 4 | 17 | 0.375 |
| (+) | 5 | 12 | |
Table 7. Relationships of ZHX2 and clinicopathological features in breast cancer.
Correlations among ZHX2, p53, and survivin
In gastric and lung cancers, most of the cases exhibited a moderate to strong positive expression of ZHX2, p53, and survivin, or at least one of them. The correlation analysis showed that in these cancer tissues, the expression of ZHX2 was positively correlated with expression of either both or one of p53 and survivin: in gastric cancer the expression of ZHX2 was positively correlated with p53 (P=0.02, r=0.376) and survivin expression (P=0.015, r=0.391), and in lung cancer, the expression of ZHX2 was positively correlated with that of p53 (P=0.000, r=0.513). The expression of ZHX2 showed no correlation with that of p53 and survivin in breast cancer.
Discussion
ZHX2 is a member of the human ZHX family, with two zincfinger structures and five homologous structure domains [13]. Presently, certain studies have shown that ZHX2 could inhibit tumor cell proliferation and AFP expression in HCC [7], inhibit tumor cell proliferation in Hodgkin lymphoma [9], and inhibit tumor diffusion in multiple myeloma [8]. Whether the abnormal ZHX2 gene expression is associated with occurrence and development of other neoplasms, and with the abnormal expression of p53 and survivin, is unknown. Accordingly, we detected the expression of ZHX2, p53, and survivin in esophageal, gastric, lung, and breast cancers to investigate the role of ZHX2 expression in cancer occurrence and development.
The results of this study showed that ZHX2 was highly expressed in the tumor tissues of esophageal, gastric, lung, and breast cancers compared to adjacent normal tissues, suggesting that ZHX2 expression is associated with the occurrence of these common cancers. As a transcriptional repressor, the normal function of ZHX2 would be to inhibit cell proliferation, suppressing tumor formation [14], but the results of this experiment revealed different phenomena. The mechanisms of how ZHX2 could promote the occurrence and development of tumors are still not clear. Previous studies have reported that although ZHX2 could inhibit the proliferation of HCC, its expression in HCC exhibiting a high TNM stage was greater than in those presenting with a low TNM stage. Expression was especially significantly increased in HCC with metastasis [15], suggesting that the functions of ZHX2 might change with the tumor progression; for example, it acts as a suppressing factor in the early stage, but becomes a contributing factor in the late stage, with the ZHX2 mechanisms now promoting the occurrence and development of tumors. Certain previous studies have shown that several transcription factors also present such functional inversion during the development of tumors.
In most cancers, tumor size is closely correlated with the malignancy degree and prognosis of the patients. In addition, many studies have shown that the expression levels of a variety of cancer-associated factors were related with tumor size [16-18] tumors with a larger volume have a higher malignant degree and present a poorer prognosis. According to the results of this study, in gastric, colorectal, lung, and breast cancers, the expression of ZHX2 was associated with the tumor size. The groups with relatively smaller tumors showed a lower ZHX2 expression, compared to those with relatively larger tumors, suggesting that in addition to promoting the occurrence of tumors, ZHX2 could also affect the tumor cell proliferation. Given the relationship between the tumor size and its malignancy degree, ZHX2 could therefore be used as an indicator for judging the malignancy degrees of tumors.
Our results showed that ZHX2 was further expressed with increasing invasion depth in esophageal and gastric cancers, while its expression was connected with the existence of regional lymph node metastasis in lung and breast cancers— the cases with regional lymph node metastasis exhibited stronger expression of ZHX2 than those without lymph node metastasis indicating that ZHX2 was closely related to the biological behaviors of the tumors. Furthermore, previous studies have shown that the expression imbalance of tissue inhibitor of metalloproteinase (TIMP) and matrix metalloproteinase (MMP) might promote tumor invasion and metastasis [19,20]. These two genes are regulated by nuclear factor YA (NF-YA), and the regulation of expression of downstream genes by ZHX2 is through its binding with NFYA [4]; therefore, it could be presumed that ZHX2 is involved in tumor invasion and metastasis by affecting the expression of downstream genes via NF-YA.
The results of this study also showed that in lung and breast cancers, in addition to the tumor size and lymph node metastasis, the expression of ZHX2 was related to the tumor differentiation degree: the cases with relatively poorly differentiated tumors exhibited stronger expression of ZHX2 than those with a higher differentiation degree. In general, the tumor differentiation degree is also related to its malignancy degree and would influence the prognosis of patients to a large extent [21]. Therefore, the results suggest that the expression of ZHX2 could reflect the malignancy degree in lung and breast cancers. As mentioned above, the tumor size was closely correlated with the expression of ZHX2 in gastric, intestinal, lung, and breast cancers. Taken together, these two results indicate the significance of ZHX2 in judging the malignancy degree of tumors.
It has been reported that p53 plays important roles in the occurrence and development of a variety of tumors, and its mutation or expression imbalance are closely associated with tumors [10]. It is believed that p53 expression is related to tumor metastasis [22]. In this experiment, all tumor tissues exhibited a higher expression of p53 compared to that in their adjacent normal tissues, supporting the relationship between p53 expression and the occurrence and development of tumors. This study also found that in gastric and lung cancers, the expressions of p53 and ZHX2 were positively correlated. Considering the relationships ZHX2 was shown to have with the invasion depth of gastric cancer, as well as with the regional lymph node metastasis of lung cancer, ZHX2 could collaborate with p53 to determine the extent of progress of these two cancers. In addition, previous studies have shown that NF-YA is involved in the expression regulation of p53 and that TobBp1 could mediate the gain-of-function mutations of p53 via NF-YA and p63/p73 [23-25]; accordingly, it could be speculated that in gastric and lung cancers, ZHX2 also regulates p53 via NF-YA, thus promoting tumor invasion and metastasis. However, this needs further experimental proof.
The anti-apoptotic factor survivin also plays an important role in the occurrence and development of tumors. This study proved that the expression of survivin in various cancer tissues were significantly higher than those in the adjacent normal tissues, and, in colorectal cancer, expression was further enhanced with the deepening of invasion. Thus, this study supports the role of survivin in promoting the occurrence and development of tumors. The correlation analysis showed that in gastric cancer and colorectal cancer, the expression of survivin and ZHX2 were positively correlated, signifying that in some cancer tissues, ZHX2 could act synergistically with p53 and survivin, or at least one of them, co-promoting the generation, invasion, and metastasis of tumors. Although previous studies also showed that, in general, survivin negatively correlated with p53, and inhibiting the expression of survivin could enhance p53 expression and increase p53- mediated apoptosis [26,27], according to the results of this study, although p53 and survivin were positively correlated with ZHX2 in some cancer tissues, p53 and survivin did not have a relationship in all cancers. In most of the cancer tissues tested, however, the highly expressed p53 had normally occurring mutations and lost the role of inhibiting the cell proliferation [28,29]; therefore, the results of this study are not contradictory against the previous findings.
In addition, the expression of ZHX2 was also related to age in breast cancer. The ZHX2 expression in the low-age group was higher than that in the relatively older group, suggesting the expression of ZHX2 might be associated with hormone secretion. While the grouping based on the expression of estrogen (ER) and progesterone (PR) showed no difference in the expression of ZHX2, it could not dismiss the existence of certain regulatory mechanisms between ZHX2, ER, and PR. Therefore, however, ZHX2 could not be considered as a potential indicator for determining whether breast cancers show signs of hormone therapy. As for the relationships of ZHX2 and age in lung cancer, survivin and gender in gastric cancer, and survivin and classification in lung cancer, because there were fewer experimental cases and no relevant literature is currently available, no practical significance could be recognized yet.
In summary, ZHX2 plays catalytic roles in the occurrence and development of common human cancers, such as esophageal, gastric, colorectal, lung, and breast cancers, and even in some cancerous tissues. A proposed mechanism is as follows: ZHX2 interacts with NF-YA and influences target genes, such as p53 and survivin. This then controls apoptosis and cell proliferation that both have roles in cancer occurrence and development when dysregulated. The relationships of ZHX2 with the invasion depth in digestive tract tumors, the regional lymph node metastasis in lung and breast cancers, and the tumor size and differentiation degree all prompted the idea that ZHX2 was related with the growth, invasion and metastasis of tumors. Therefore, ZHX2 could be used an indicator to judge the malignancy degree and analyze the biological behaviors of tumors. In the future, ZHX2 could be expected to become a diagnostic indicator for the above cancers, and to provide new therapeutic targets in gene-targeting treatment of cancers.
Acknowledgement
This study was supported by Scientific and technological research projects in Guangxi Universities, KY2015YB065 and Guangxi Youth Scientific Foundation (No.0728057).
References
- Han T, Xiang DM, Sun W, Liu N, Sun HL, Wen W, Shen WF, Wang RY, Chen C, Wang X, Cheng Z, Li HY, Wu MC, Cong WM, Feng GS, Ding J, Wang HY. PTPN11/Shp2 overexpression enhances liver cancer progression and predicts poor prognosis of patients. J Hepatol 2015; 63: 651-660.
- Yu X, Li Z, Chan MT, Wu WK. PAQR3: a novel tumor suppressor gene. Am J Cancer Res 2015; 5: 2562-2568.
- Santo L, Siu KT, Raje N. Targeting cyclin-dependent kinases and cell cycle progression in human cancers. Semin Oncol 2015; 42: 788-800.
- Yu X, Li Z, Chan MT, Wu WK. PAQR3: a novel tumor suppressor gene. Am J Cancer Res 2015; 5: 2562-2568.
- Weng MZ, Zhuang PY, Hei ZY, Lin PY, Chen ZS, Liu YB, Quan ZW, Tang ZH. ZBTB20 is involved in liver regeneration after partial hepatectomy in mouse. Hepatobiliary Pancreat Dis Int 2014; 13: 48-54.
- Luan F, Liu P, Ma H, Yue X, Liu J, Gao L, Liang X, Ma C. Reduced nucleic ZHX2 involves in oncogenic activation of glypican 3 in human hepatocellular carcinoma. Int J Biochem Cell Biol 2014; 55: 129-135.
- Perincheri S, Dingle RW, Peterson ML, Spear BT. Hereditary persistence of alpha-fetoprotein and H19 expression in liver of BALB/cJ mice is due to a retrovirus insertion in the ZHX2 gene. Proc Natl Acad Sci USA 2005; 102: 396-401.
- Armellini A, Sarasquete ME, García-Sanz R, Chillón MC, Balanzategui A, Alcoceba M, Fuertes M, López R, Hernández JM, Fernández-Calvo J, Sierra M, Megido M, Orfão A, Gutiérrez NC, González M, San Miguel JF. Low expression of ZHX2, but not RCBTB2 or RAN, is associated with poor outcome in multiple myeloma. Br J Haematol 2008; 141: 212-215.
- Nagel S, Schneider B, Meyer C, Kaufmann M, Drexler HG, Macleod RA. Transcriptional deregulation of homeobox gene ZHX2 in Hodgkin lymphoma. Leu Res 2012; 36: 646-655.
- Meek DW. Regulation of the p53 response and its relationship to cancer. Biochem J 2015; 469: 325-346.
- Pluta P, Jeziorski A, Cebula-Obrzut AP, Wierzbowska A, Piekarski J, Smolewski P. Expression of IAP family proteins and its clinical importance in breast cancer patients. Neoplasma 2015; 62: 666-673.
- Senol S, Yildirim A, Ceyran B, Uruc F, Zemheri E, Ozkanli S, Akalin I, Ulus I, Caskurlu T, Aydin A. Prognostic significance of surviving, β-catenin and p53 expression in urothelial carcinoma. Bosn J Basic Med Sci 2015; 15: 7-14.
- Kawata H, Yamada K, Shou Z, Mizutani T, Yazawa T, Yoshino M, Sekiguchi T, Kajitani T, Miyamoto K. Zinc-fingers and homeoboxes (ZHX) 2, a novel member of the ZHX family, functions as a transcriptional repressor. Biochem J 2003; 373: 747-757.
- Nakao K, Ichikawa T. Recent topics on α-fetoprotein. Hepatol Res 2013; 43: 820-825.
- Hu S, Zhang M, Lv Z, Bi J, Dong Y, Wen J. Expression of zinc-fingers and homeoboxes 2 in hepatocellular carcinogenesis: a tissue microarray and clinicopathological analysis. Neoplasma 2007; 54: 207-211.
- Liu FY, Deng YL, Li Y, Zeng D, Zhou ZZ, Tian DA, Liu M. Down-regulated KLF17 expression is associated with tumor invasion and poor prognosis in hepatocellular carcinoma. Med Oncol 2013; 30: 425.
- Li SJ, Wang WY, Li B, Chen B, Zhang B, Wang X, Chen CS, Zhao QC, Shi H, Yao L. Expression of NDRG2 in human lung cancer and its correlation with prognosis. Med Oncol 2013; 30: 421.
- Miao X, Yang ZL, Xiong L, Zou Q, Yuan Y, Li J, Liang L, Chen M, Chen S. Nectin-2 and DDX3 are biomarkers for metastasis and poor prognosis of squamous cell/adenosquamous carcinomas and adenocarcinoma of gallbladder. Int J Clin Exp Pathol 2013; 6: 179-190.
- Deryugina EI, Zajac E, Juncker-Jensen A, Kupriyanova TA, Welter L, Quigley JP. Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia 2014; 16: 771-788.
- Yang HK, Jeong KC, Kim YK, Jung ST. Role of matrix metalloproteinase (MMP) 2 and MMP-9 in soft tissue sarcoma. Clin Orthop Surg 2014; 6: 443-454.
- Fu J, Qiu H, Cai M, Pan Y, Cao Y, Liu L, Yun J, Zhang CZ. Low cyclin F expression in hepatocellular carcinoma associates with poor differentiation and unfavorable prognosis. Cancer Sci 2013; 104: 508-515.
- Hu S, Cao B, Zhang M, Linghu E, Zhan Q, Brock MV, Herman JG, Mao G, Guo M. Epigenetic silencing BCL6B induced colorectal cancer proliferation and metastasis by inhibiting P53 signaling. Am J Cancer Res 2015; 5: 651-662.
- Tue NT, Yoshioka Y, Yamaguchi M. NF-Y transcriptionally regulates the Drosophila p53 gene. Gene 2011; 473: 1-7.
- Imbriano C, Gnesutta N, Mantovani R. The NF-Y/p53 liaison: Well beyond repression. Biochim Biophys Acta 2012; 1825: 131-139.
- Liu K, Ling S, Lin WC. TopBP1 mediates mutant p53 gain of function through NF-Y and p63/p73. Mol Cell Biol 2011; 31: 4464-4481.
- Huang KF, Zhang GD, Huang YQ, Diao Y. Wogonin induces apoptosis and down-regulates survivin in human breast cancer MCF-7 cells by modulating PI3K-AKT pathway. Int Immunopharmacol 2012; 12: 334-341.
- Tyner JW, Jemal AM, Thayer M, Druker BJ, Chang BH. Targeting survivin and p53 in pediatric acute lymphoblastic leukemia. Leukemia 2012; 26: 623-632.
- Turner N, Moretti E, Siclari O, Migliaccio I, Santarpia L, D'Incalci M, Piccolo S, Veronesi A, Zambelli A, Del Sal G, Di Leo A. Targeting triple negative breast cancer: is p53 the answer? Cancer Treat Rev 2013; 39: 541-550.
- Guo G, Marrero L, Rodriguez P, Del Valle L, Ochoa A, Cui Y. Trp53 inactivation in the tumor microenvironment promotes tumor progression by expanding the immunosuppressive lymphoid-like stromal network. Cancer Res 2013; 73: 1668-1675.