Research Article - Biomedical Research (2017) Volume 28, Issue 8
Content determination in Prunella vulgaris L. and its anti-proliferative effect on thyroid cancer cells
Ying Ba and Yulin Wang*The First Affiliated Hospital of Dalian Medical University, Dalian, PR China
- *Corresponding Author:
- Yulin Wang
The First Affiliated Hospital of Dalian Medical University, PR China
Accepted on December 2, 2016
Abstract
To establish the method for determination of chlorogenic and caffeic acid contents in Prunella vulgaris L., and to investigate the anti-proliferative effect of Prunella vulgaris L. extract on thyroid cancer cells. With chlorogenic and caffeic acids as the quantitative determination targets, their contents in Prunella vulgaris L. are determined by HPLC. Chromatographic column used is Kromasil C18 (250 mm × 4.6 mm, 5 μm), and gradient elution mode is employed. Mobile phase: A is 0.5% phosphoric acid in methanol, and B is acetonitrile, gradient elution: 0~45 min 5%~10% (acetonitrile); flow rate is 1.0 ml/ min; detection wavelength is 323 nm; and column temperature is 20°C. After 48 h treatment of thyroid cancer K1 cells with different concentrations of Prunella vulgaris L. extract, half maximal inhibitory concentration (IC50) of Prunella vulgaris L. extract against K1 cells is calculated by MTT assay. After 48 h treatment of K1 cells with drugs in various groups, morphological changes of K1 cells are observed under inverted phase contrast microscope. Expression of c-myc protein in each group is detected by Western blot. Linear range of chlorogenic acid is 0.32~1.6 μg, r=0.9996, while linear range of caffeic acid is 0.48~2.4 μg, r=0.9998. Sample recovery is 97.6%, RSD=1.5% for chlorogenic acid, and 96.6%, RSD=1.6% for caffeic acid. IC50 of Prunella vulgaris L. extract against K1 cells is 2 mg/ml. Expression of c-myc protein is significantly lower in Prunella vulgaris L. extract groups than in the negative control group (P?0.05). The method proposed herein is accurate, simple and fast, which is suitable for quantitative analysis of Prunella vulgaris L. Prunella vulgaris L. has rather marked inhibitory effect on proliferation of thyroid cancer cells, which provides a theoretical basis for its use in the treatment of thyroid cancer.
Keywords
Prunella vulgaris L., HPLC, Chlorogenic acid, Caffeic acid, Quantitative determination.
Introduction
Prunella vulgaris L. is an herbaceous plant in the genus Prunella of the family Lamiaceae; generally, the part used as medicine is its dried fruits. Prunella vulgaris L. is a common Chinese herbal medicine with a definite curative effect. Flavonoids, as its active constituent, have important pharmacological actions and healthcare functions, which have a wide range of uses in the pharmaceutical, food, household chemical and other related industries. Prunella vulgaris L. mainly contains triterpenoids and their glycosides, flavonoids, sterols and their glycosides, coumarin, organic acids, volatile oils, saccharides, etc. [1-3]. Modern studies have found that Prunella vulgaris L. has a variety of activities such as anti- HIV, immunomodulatory and antioxidant effects [4-6]. With chlorogenic and caffeic acids as the quantitative determination targets, this paper determines their contents in Prunella vulgaris L. The method proposed is simple and reproducible, which can be used for quantitative analysis of Prunella vulgaris L., thus providing the theoretical basis for rational exploitation of its resources and its quality control.
The search for drugs with anti-tumor activity from natural medicine has become a hot topic in the research of tumor treatment in recent years. Prunella vulgaris L. has liver clearing, vision improving, hard lump softening and stagnation dissipating effects, which is commonly used for headache, dizziness, hypertension, breast swelling, acute mastitis, breast cancer, goitre and lymph node tuberculosis; it is especially effective for lymphatic system tumors [7]. Proto-oncogene cmyc encodes DNA-binding proteins with transcriptional regulatory effects, is involved in the regulation of cellular metabolism, proliferation, differentiation, adhesion and apoptosis processes, and is also related to the development and progression of human tumors [8]. Overexpression of c-myc is a characteristic of many tumors. This study investigates the antiproliferative effect of Prunella vulgaris L. on thyroid cancer K1 cells, as well as the intracellular expression of c-myc protein in Prunella vulgaris L.-treated K1 cells, thereby providing the theoretical basis for its use in the treatment of thyroid cancer.
Instruments and Reagents
Instruments
HP-1110 series HPLC system (HP-1110 DAD detector; HP Chemstation); TPC-1204 electronic balance (Nanjing Precision Instrument Factory); CSB-652 ultrasonic cleaner (Jiangsu Ultrasonic Instrument Factory).
Reagents
Chlorogenic and caffeic acid reference substances were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (batch No.: 201402154, 201410254, respectively). Prunella vulgaris L. was purchased from Guangzhou Baiyuntang Medicine Company, which was identified by Professor Wang Minyuan at China Pharmaceutical University as Prunella vulgaris L. HPLC grade acetonitrile and methanol were purchased from Tianjin Kemiou Chemical Reagent Co., Ltd. Analytical grade phosphoric acid, ultra-pure water (self-prepared). Other reagents were of analytical grade.
Thyroid cancer K1 cells were provided by China Medical University. Preparation of Prunella vulgaris L. extract: 100 g of dried Prunella vulgaris L. was taken, added with 250 ml of water, boiled for 30 min, and then filtered to remove residues. The remaining was autoclaved at 120°C for 30 min, and then filtered under positive pressure through a 0.22 μL diameter microporous membrane. During experiment, L-15 medium was used to dilute the extract to desired concentrations. L-15 medium was purchased from Nanjing Biologics Company; FBS was purchased from Jiangsu Qingsong Biology Company; trypsin, DMSO and MTT were purchased from Nanjing Sikate Reagent Company; rabbit anti-human c-myc antibody was purchased from Cell Signaling; horseradish peroxidaselabelled goat anti-rabbit IgG was purchased from Santa Cruz Biotechnology; internal reference β-actin was purchased from Tianjin Sihu Biologics Co., Ltd.; vertical electrophoresis chamber and electrophoretic transfer cell were purchased from Bio-Rad; inverted phase contrast microscope was purchased from Carl Zeiss, Germany; and microplate reader was purchased from Biotek, USA.
Methods
Chromatographic conditions
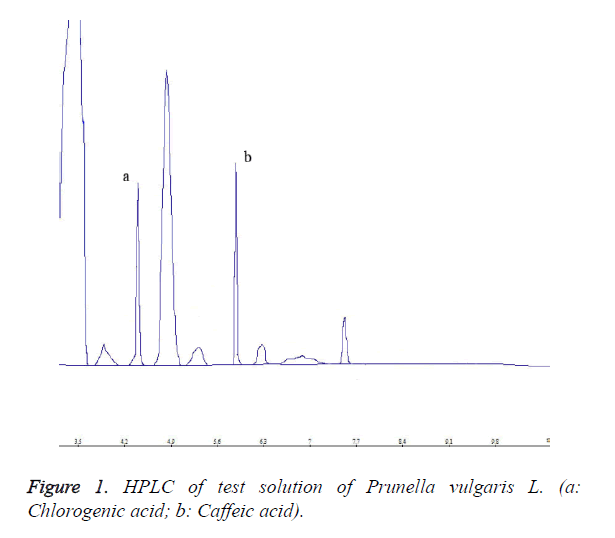
Chromatographic column was Kromasil C18 (250 mm × 4.6 mm, 5 μm), continuous gradient elution was performed with acetonitrile~methanol (5%~10%); elution time was 45 min; flow rate was 1.0 ml/min; detection wavelength was 323 nm; and column temperature was 20°C.
Preparation of reference solutions
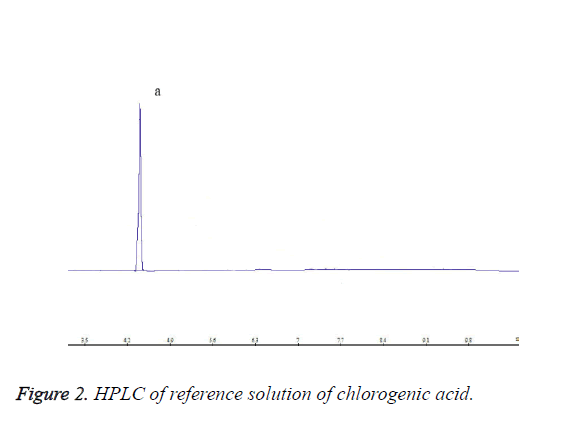
Appropriate amount of chlorogenic acid reference substance was accurately weighed, and added with 50% methanol to prepare an 80 μg/ml chlorogenic acid reference stock solution.
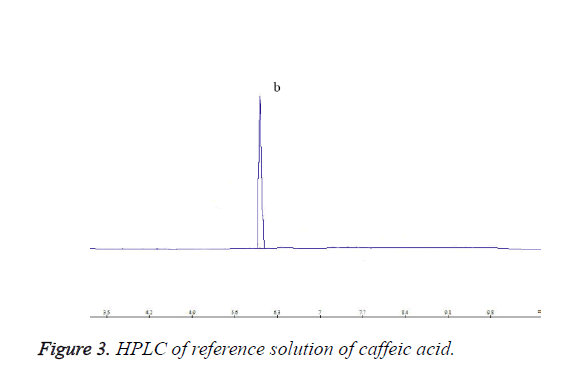
Meanwhile, appropriate amount of caffeic acid reference substance was accurately weighed, and added with 50% methanol to prepare a 120 μg/ml caffeic acid reference stock solution.
Preparation of test solution
2 g of dry Prunella vulgaris L. powder was accurately weighed, placed in a 250 ml round bottom flask, added with 50 ml of 50% methanol and soaked for 1 h, then extracted under reflux for 2 h. After letting cool and filtration, the extract was washed with 50% methanol 2 or 3 times, and then evaporated to dryness. Next, the residue was added with appropriate amount of chloroform and soaked for 10 min, then chloroform solution was discarded, chloroform was evaporated, and the remaining was dissolved and diluted to the mark in a 50 ml volumetric flask with 50% methanol. Finally, 2.5 ml of the solution was drawn, and diluted to the mark with 50% methanol in a 10 ml volumetric flask.
System suitability test
Each 10 μL of chlorogenic acid reference solution, caffeic acid reference solution and test solution was precisely drawn, and injected into the HPLC system for determination. HPLC chromatograms are shown in Figures 1-3.
Investigation of sample extraction method
Investigation of sample extraction solvent: Three aliquots of 2 g of dry Prunella vulgaris L. powder were accurately weighed, added separately with 250 ml of 50% methanol and water, and ultrasonically extracted for 40 min. After filtration through microporous membrane (0.45 μm), each 10 μl of the extract was injected for determination. The results showed that the chlorogenic and caffeic acid contents were maximum when 50% methanol was used as the extraction solvent, so 50% methanol was selected as the extraction solvent.
Investigation of different extraction methods: Three aliquots of 2 g of dry Prunella vulgaris L. powder were accurately weighed, placed in 150 ml round bottom flasks, added with 50 ml of 50% methanol, and extracted separately by reflux, Soxhlet and ultrasonic extraction methods. After filtration, the extracts were washed with 50% methanol 2 or 3 times, and then evaporated to dryness. Next, the residues were added with appropriate amount of chloroform and soaked for 10 min, then chloroform solution was discarded, chloroform was evaporated, and the remaining was dissolved with 50% methanol and diluted to the mark in 250 ml volumetric flasks. Finally, the solutions were filtered through microporous membrane (0.45 μm), and then injected at a volume of 10 μL for determination. The results showed that the chlorogenic and caffeic acid contents were the highest when reflux extraction was used, so reflux extraction was selected as the extraction method.
Investigation of extract time and frequency: Four aliquots of 2 g of dry Prunella vulgaris L. powder were accurately weighed, respectively, prepared into test solutions as per the method under “Preparation of test solution”, and then injected for HPLC determination. The results revealed that the chlorogenic and caffeic acid contents were the highest when extraction was performed for 2 h once, so 2 h and once were selected as the extraction time and frequency.
Investigation of linearity
Investigation of linearity of chlorogenic acid: 4, 8, 12, 16 and 20 μL of chlorogenic acid reference solution were precisely drawn, respectively, and injected into the HPLC system for determination of peak area. Standard curve was plotted with chlorogenic acid amount (μg) as abscissa and chlorogenic acid peak area as ordinate. Its regression equation was Y=2245.6 X-11.231, r=0.9996. The results indicated that chlorogenic acid had a good linearity within 0.32~1.6 μg.
Investigation of linearity of caffeic acid: 4, 8, 12, 16 and 20 μL of caffeic acid reference solution were precisely drawn, respectively, and injected into the HPLC system for determination of peak area. Standard curve was plotted with caffeic acid amount (μg) as abscissa and caffeic acid peak area as ordinate. Its regression equation was Y=4232.7 X-40.122, r=0.9998. The results indicated that caffeic acid had a good linearity within 0.48~2.4 μg.
Accuracy test
2 g of dry Prunella vulgaris L. powder was accurately weighed, and prepared into the test solution as per the above method. 10 μL of the test solution was drawn, and injected five consecutive times for determination of peak area. The results revealed that RSD=1.6% for chlorogenic acid, and RSD=1.4% for caffeic acid, suggesting good accuracy.
Reproducibility test
Five aliquots of 2 g of dry Prunella vulgaris L. powder were accurately weighed, respectively, and prepared into the test solutions as per the above method. Each 10 μL of the test solutions was accurately drawn, and injected for determination of peak area. The results revealed that RSD=2.6% for chlorogenic acid, and RSD=2.1% for caffeic acid, indicating good reproducibility of the proposed method.
Stability test
Five aliquots of 2 g of dry Prunella vulgaris L. powder were accurately weighed and prepared into the test solutions as per the above method. Each 10 μL of the test solutions was accurately drawn at 0, 6, 12, 18 and 24 h, respectively, and injected for determination of peak area. The results revealed that RSD=1.8% for chlorogenic acid, and RSD=1.4% for caffeic acid, indicating that the test solution was basically stable within 24 h.
Sample recovery test
Five aliquots of 1 g of dry Prunella vulgaris L. powder (containing 0.8123% chlorogenic acid, and 1.2986% caffeic acid) were accurately weighed. Each 5 μL of 80 μg/ml chlorogenic acid reference solution and 120 μg/ml caffeic acid reference solution was precisely drawn, and injected for HPLC determination. The results are shown in Tables 1 and 2.
| No. | Content in sample (mg) | Measured amount (mg) | Recovery (%) | Average recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| 1 | 0.4062 | 0.8101 | 100.98 | ||
| 2 | 0.4065 | 0.8024 | 98.98 | ||
| 3 | 0.4062 | 0.8075 | 100.33 | 99.01 | 2.02 |
| 4 | 0.407 | 0.7901 | 95.78 | ||
| 5 | 0.4071 | 0.8031 | 99 | ||
| Note: Added amount of chlorogenic acid reference substance is all 0.4 mg | |||||
Table 1. Results of chlorogenic acid recovery test.
| No. | Content in sample (mg) | Measured amount (mg) | Recovery (%) | Average recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| 1 | 0.6012 | 1.2001 | 99.82 | 99.22 | 0.99 |
| 2 | 0.6213 | 1.2243 | 100.50 | ||
| 3 | 0.6092 | 1.2035 | 99.05 | ||
| 4 | 0.6122 | 1.1998 | 97.93 | ||
| 5 | 0.6106 | 1.2033 | 98.78 | ||
| Note: Added amount of caffeic acid reference substance is all 0.1818 mg | |||||
Table 2. Results of caffeic acid recovery test.
The above results indicated that the proposed method had a good recovery.
Determination of sample content
Four batches of samples were taken, prepared as per the test sample method, and determined. The results are shown in Table 3.
| Sample no. | Chlorogenic acid content | Caffeic acid content |
|---|---|---|
| 1 | 0.8123 | 1.2023 |
| 2 | 0.8018 | 1.2041 |
| 3 | 0.8110 | 1.1908 |
| 4 | 0.7998 | 1.1998 |
| Mean | 0.8062 | 1.1993 |
Table 3. Sample content determination results (mg/g).
Study of anti-thyroid cancer activity of Prunella vulgaris L.
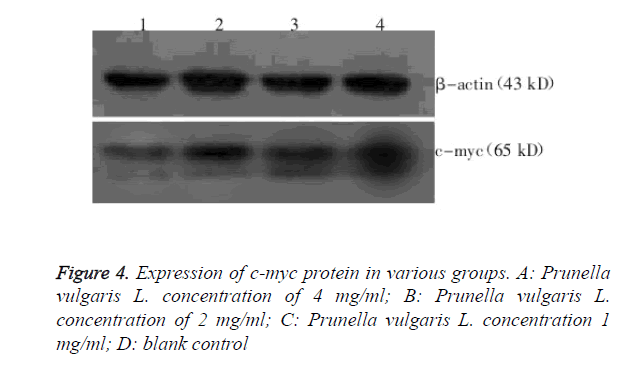
Cell culturing and experimental grouping: Human thyroid cancer K1 cells were cultured in10% FBS containing L-15 medium, and incubated in a 37°C incubator without CO2. The experiment consists of four groups: 1. Prunella vulgaris L. high concentration group (with Prunella vulgaris L. extract concentration of 4 mg/ml); 2. Prunella vulgaris L. medium concentration group (with Prunella vulgaris L. extract concentration of 2 mg/ml); 3. Prunella vulgaris L. low concentration group (with Prunella vulgaris L. extract concentration of 1 mg/ml); and 4. Negative control group (with medium containing cells only without drug).
IC50 of drugs
Logarithmic phase cells were digested, made into single cell suspension, and seeded in 96 well plates at a density of 8 × 104 cells/ml at 100 μL/well. After culturing for 24 h, different concentrations of Prunella vulgaris L. extracts were added into the cells, respectively. 48 h later, cellular morphology was observed under inverted phase contrast microscope. Then the cells were carefully washed twice with PBS, and 100 μL complete medium and 10 μL of 5 mg/ml MTT were added to each well. After culturing for an additional 4 h, supernatant was carefully discarded, 100 μL of DMSO was added to each well, and the plates were shaken at a low speed on an automatic microplate reader for 10 min to completely dissolve the crystals. After the reader was zeroed with DMSO, absorbance (A) value was measured at 490 nm, and cell proliferation inhibition rate was calculated by the following formula: Cell proliferation inhibition rate=(1-A value of experimental group/A value of control group) × 100%. Finally, IC50 of the two drugs were calculated.
Determination of cell proliferation inhibition rate by MTT assay
Cell proliferation inhibition rates in Prunella vulgaris L. extract groups and negative control group after 48 h of drug treatment were determined by MTT assay, and growth state of cells was observed under inverted phase contrast microscope.
Detection of c-myc expression by western blot
Cells in each group were collected by centrifugation, respectively. Approximately 1 × 106 cells were added with 100 L of lysis buffer, and lysed by pipetting on ice for 30 min, then centrifuged at 4°C, 12000 r/min for 30 min. The supernatants were carefully collected as the total cellular protein extracts. Protein concentrations were determined, based on which the extracted were loaded for SDS-PAGE two dimensional gel electrophoresis. Proteins were electrophoretically transferred from the gel to nitrocellulose membrane for Western blot analysis with rabbit anti-human c-myc antibody as the first antibody, horseradish peroxidase-labelled goat anti-rabbit IgG as the secondary antibody, and β-actin as the internal reference. Quantitative analysis was performed using Quantity-one image analysis software.
Statistical processing
Data were analysed using SPSS 13.0 statistical software, and expressed as (x ± s). Comparison among groups was performed by one-way ANOVA, and pairwise comparison was done by LSD method for each set of data pairwise comparison. α=0.05 was taken as the significance level, and p<0.05 was considered significantly different.
Results
IC50 of Prunella vulgaris L. against thyroid cancer
IC50 of Prunella vulgaris L. against K1 cells was determined by MTT assay to be 2 mg/ml.
Effect of each treatment group on cell proliferation
Cell proliferation inhibition rates of different concentrations of Prunella vulgaris L. were determined by MTT assay. Cell proliferation inhibition rates of Prunella vulgaris L. groups and control group were (49.04 ± 5.18%), (11.70 ± 4.50%), (57.60 ± 6.13%) and 0, respectively. Prunella vulgaris L. extracts with concentrations of 4 mg/ml and 2 mg/ml could markedly inhibit the proliferation of K1 cells, and the inhibitory effects were significantly different compared with the control group (P˂0.05).
Morphological changes of K1 cells
Growth state of cells was observed under inverted phase contrast microscope. After treatment with 4 mg/ml and 2 mg/ml Prunella vulgaris L. extracts, intercellular space increased, part of cells had cytoplasmic retraction and rounding, cell junction disappeared, and cytoplasm shrank, presenting apoptosis.
Expression levels of c-myc protein in various treatment groups
Expression levels of c-myc protein in K1 cells of various treatment groups were determined by Western blot, as shown in Figure 4. Relative c-myc protein expression level was (0.58 ± 0.04) for 4 mg/ml Prunella vulgaris L.; (0.81 ± 0.01) for 2 mg/ml Prunella vulgaris L.; (0.96 ± 0.03) for 1 mg/ml Prunella vulgaris L.; and (0.97 ± 0.03) for the control group. 4 mg/ml Prunella vulgaris L. group had c-myc protein expression levels all significantly lower than the control group (P<0.05).
Discussion
This paper studies the quantitative determination of Prunella vulgaris L.; experimental results show that the method proposed herein is simple, accurate and reproducible, which can effectively control the intrinsic quality of Prunella vulgaris L. and related preparations.
Thyroid cancer is a relatively common tumor, which has now become one of the fastest growing cancers in terms of incidence [9]. At present, thyroid cancer is treated primarily by surgery, but postoperative recurrence and metastasis are highly likely. As a new therapeutic approach for thyroid cancer, TCM therapy has become a hot research topic in tumor adjuvant therapy in recent years.
Literature has reported that Prunella vulgaris L. has varying degree of inhibitory effects on a variety of tumors [10]. This study uses MTT assay to observe the proliferation inhibitory effect of Prunella vulgaris L. on K1 cells. The results show that Prunella vulgaris L. can inhibit the growth of K1 cells. Under microscope, most K1 cells presented cytoplasmic retraction, increased intercellular space and apoptosis, suggesting that Prunella vulgaris L. can inhibit cell proliferation; such inhibitory effect may be associated with apoptosis.
This study finds that the K1 cell proliferation inhibitory effect between low-dose (1 mg/ml) Prunella vulgaris L. group and control group is not significantly different (P>0.05), which indicates that Prunella vulgaris L. almost has no proliferation inhibitory effect at that concentration. Cell proliferation inhibitory effects of 4 mg/ml and 2 mg/ml Prunella vulgaris L. are more evident than the 1 mg/ml Prunella vulgaris L., which are significantly different compared with the control group (P<0.05). Moreover, marked changes in cell morphology are observed under microscope, such as cytoplasmic retraction and rounding, disappearance of cell junction and presence of apoptosis, indicating that the anti-tumor activity is enhanced after thyroid cancer cells are treated with medium- and highdose Prunella vulgaris L. This suggests that medium- and high-dose Prunella vulgaris L. have anti-proliferative effects on thyroid tumors.
In recent years, tumor studies have shown that the activation of oncogenes and deactivation of tumor suppressor genes can lead to the occurrence and development of a variety of tumors. c-myc is a nucleoprotein type regulator gene; relevant research has shown that c-myc proto-oncogene is activated mainly by amplification and overexpression of that gene [11]. When its expression is high, it can promote the proliferation and malignant transformation of cells. Meanwhile, as an important transcriptional regulator, c-myc protein has a two-way regulatory effect [12], which mediates cell proliferation or apoptosis based on the types of signals cells receive and the growth environment of cells. This study finds that c-myc protein is highly expressed in K1 cells. Western blot results show that c-myc protein expression is lower in Prunella vulgaris L. medium- and high-dose groups than in the negative control group; the down-regulation of c-myc protein expression in Prunella vulgaris L. medium- and high-dose groups is consistent with the cell proliferation inhibitory effect. According to a literature report [13], suppression of c-myc gene expression can lead to tumor regression, which may be associated with apoptosis. This study finds that after treatment of K1 cells with high-dose Prunella vulgaris L., cells present rather typical apoptosis, as well as more marked c-myc protein expression than the medium-dose Prunella vulgaris L. group.
At present, anti-tumor effect of traditional Chinese medicine is achieved mainly through the induction of tumor cell apoptosis. Medium and high doses of Prunella vulgaris L. can resist the proliferation of thyroid cancer cells, which is probably achieved by induction of tumor cell apoptosis by inhibiting c-myc protein expression. Occurrence and development of tumors are associated with a variety of factors, and cell proliferation and apoptosis are also participated and regulated by multiple factors. Whether this process involves activation of other apoptosis genes needs further research.
References
- Liu JP, Feng L, Gu JF, Wang RS, Zhang MH, Jiang J, Qin D, Jia XB, Chen Y, Chen SX, Sataer R, Zhang X, Zhu MM. Simultaneous determination of ten characteristic antioxidant compounds for inhibiting cancer cell proliferation in Prunella vulgaris L. from different regions using HPLC-UV coupled with MS identification. Anal Methods 2014; 6: 3139-3146.
- Lee IK, Kim DH, Lee SY, Kim KR, Choi SU, Hong JK, Lee JH, Park YH, Lee KR. Triterpenoic acids of Prunella vulgaris var. lilacina and their cytotoxic activities in vitro. Arch Pharm Res 2008; 31: 1578-1583.
- Sahin S, Demir C, Malyer H. Determination of phenolic compounds in Prunella L. by liquid chromatography-diode array detection. J Pharm Biomed Anal 2011; 55: 1227-1230.
- Oh C, Price J, Brindley MA, Widrlechner MP, Qu L. Inhibition of HIV-1 infection by aqueous extracts of Prunella vulgaris L. Virol J 2011; 8: 188.
- Park KH, Choi S. Effects of Prunella vulgaris labiatae extract on specific and non-specific immune responses in tilapia (Oreochromis niloticus). J Animal Sci Technol 2014; 56: 3-9.
- Zhang GW, He L, Hu MM. Optimized ultrasonic-assisted extraction of flavonoids from Prunella vulgaris L. and evaluation of antioxidant activities in vitro. Innov Food Sci Emerg Technol 2011; 12: 18-25.
- Feng L, Jia XB, Shi F, Chen Y. Identification of two polysaccharides from Prunella vulgaris L. and evaluation on their anti-lung adenocarcinoma activity. Molecules 2010; 15: 5093-5103.
- Dang CV, ODonnell KA, Zeller KI, Nguyen T, Osthus RC. The c-Myc target gene network. Semin Cancer Biol 2006; 16: 253-264.
- Regional Thyroid Cancer Group. Northern Cancer Network Guidelines for Management of Thyroid Cancer. Clinical Oncol 2000; 12: 373-391.
- Hatakeyama S, Watanabe M, Fujii Y, Nakayama KI. Targeted destruction of c-myc by an engineered ubiquitin ligase sup-presses cell transformation and tumor formation. Cancer Res 2005; 65: 7874-7879.
- Xia B, Tian C, Guo SQ, Zhang L, Zhao DD, Qu FL, Zhao WP, Wang YF, Wu XX, Da WM, Wei S, Zhang YZ. c-Myc plays part in drug resistance mediated by bone marrow stromal cells in acute myeloid leukemia. Leukemia Res 2015; 39: 92-99.
- Patel JH, McMahon SB. Targeting of Miz-1 is essential for Myc-mediated apoptosis. J Biol Chem 2006; 281: 3283-3289.
- Wu CH, Riggelen JV, Yetil A, Fan AC, Bachireddy P, Felsher DW. Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation. Proc Natl Acad Sci 2007; 104: 13028-13033.