Research Article - Journal of Environmental Waste Management and Recycling (2021) Volume 4, Issue 1
Conditions Optimization And Physiochemical Analysis Of Oil Obtained By Catalytic Pyrolysis Of Scrap Tube Rubber Using MgO As Catalyst
Riaz Muhammad1,2,3*, Ali Riaz2,4
1University of Rostock, D-18029 Rostock, Germany
2Sarhad University of Science & Information Technology, Peshawar 25000, Pakistan
3Leibniz-Institute for Catalysis at the University of Rostock, D-18059 Rostock, Germany
4Centre for Optics, Photonics and Laser, University of Laval, 2375 rue de la Terrasse, Quebec (QC),G1V0A6, Canada
- Corresponding Author:
- Riaz Muhammad
University of Rostock
Germany
E-mail: riazm.chemistry@suit.edu.pk
Accepted date: January 15, 2021
Citation: Muhammad R, Riaz A. Conditions optimization and physiochemical analysis of oil obtained by catalytic pyrolysis of scrap tube rubber using MgO as catalyst.. Environ Waste Management Recycling. 2021;4(1):1-6.
Abstract
Motor vehicles scrap tires and tube rubbers generate a large amount of waste with different characteristics and compositions, contaminating the environment when not properly disposed. Waste inner tube rubber (isobutylene isoprene) representing a threat to the environment can be used as source of energy and valuable. Waste inner tube rubber was pyrolysed thermally under atmospheric pressure both with and without catalyst. Parameters of temperature, time and catalyst weight were optimized for catalytic and thermal pyrolsis of isobutylene-isoprene rubber into liquid fuel, using MgO as catalyst. It was found that one-hour heating time at 350°C using 2g catalyst (MgO) are suitable parameter for maximum conversion of scrap inner tube rubber into oil. The oil obtained was characterized by physical and chemical tests. Among the physical test Density, Specific gravity, Viscosity, Kinematic viscosity, Analine point and Flash point were determined according to IP and ASTM standard valves. The physical tests indicate the presence of aromatic and olefinic hydrocarbons. Among the chemical tests Phenol test, Bromine number, Bromine water test and KMnO4 tests were conducted. The chemical tests are also the support of physical tests conducted. The physical and chemical tests indicate that the oil obtained is a mixture of kerosene, diesel and light oil and could be used for fuel purposes.
Keywords
Catalytic pyrolysis, Waste tube rubber, Liquid fuel, Magnesium oxide.
Introduction
The surface of the product. The carbonization was optimized at 500oC along with activation temperature at 900oC yielding sorbent with a surface area of 473 m2/g.
B.C. Yu and fellows reported low cost electrochemical means of recycling waste rubber. D. Czajczynska and co-workers [1] reviewed wasted tires dumps as habitats for mosquitos and rodents that are associated with many diseases, hence are a threat to the human health as well as environment. They swotted that waste tire can be recycled to a useful gaseous fuel so as the mentioned risks to human health as well as to environment can be reduced. R. Vihar and his fellows [2] investigated the use of pyrolysis oil produced from waste tires in a turbocharged heavy-duty engine, where they reported that their product oil can resourcefully be used as a fuel in a turbocharged non-intercooled engine. Their findings suggest the use of pyrolyzed oil for power generation. S. Luo and Y. Feng [1] utilized the heat of blast-furnace slag as waste energy Wasted inner tube is the type of synthetic rubber, that have the capability of retaining air, is mostly used by the automobile and alike industry. It is a co-polymer of 97%-98% isobutylene and 2%-3% isoprene prepared at low temperature (100oC) via Friedel-Craft’s catalyst. The combination of various factors like resistance to oxidation, ozone, bacteria and solar radiation makes Butyl Rubber (BR) a good material for making tyre as well as inner tube by automobile industry. Regular butyl rubber is most preferably used material for inner tubes in a range of lightweight to lifestyle bicycles as well as daily used to luxury motorcycles.
The development of Butyl rubber can be traced back to the work of Gorianov and Butlerov in early 1870’s, and later Otto (1927) in an attempt to yield low molecular weight oily polymers by isobutylene polymerization at room temperature in the presence of sulfuric acid and BF3. Later on, in 1930, I.G. Farben workers succeeded in producing higher molecular weight polymers by reacting a dilute hydrocarbon solution of isobutylene with BF3 at very low temperatures. In a similar fashion a variety of Lewis acids can be used to initiate such kind of polymerization. [1,2]. Lin [3] reported the conversion of scrape Styrene-Butadiene Rubber (SBR), thermogravimetrically (temperature range of 127°C-677°C) under nitrogen atmosphere, in to a useful energy source. These results were supported by Miguel Miranda and his co-workers [4] while exploring the effect of temperature on the mechanism of tyre waste’s pyrolysis. They entrenched the effect of temperature on pyrolysis reactions of tyre wastes.
R. Edwin Raj and his fellow researchers [5] used fluidized bed combustion system where they reported that slower feed rate at 440oC may increase the residence time in reactor for maximum oil yield. They investigated physical and chemical properties of product oil (pyrolysis as well as distilled oil) along with the evolved gases during the process to assess the suitability of their product as conventional fuel. Telnov and his co-workers [6] studied the degradation of butyl rubber via electron beam at 6 MeV to 10 MeV. The degraded material was re-used to formulate the diaphragm for fabric and roofing purpose. M. En and his co-workers [7] reported the use of gamma irradiation for degrading iso-butylene-isoprene rubber where the effect of dose rate on degradation was studied. Their findings indicate that samples were better degraded at lower dose rate and under oxidizing atmosphere compared to the degradation at higher dose rate and under nitrogen atmosphere. Perera [8] investigated gamma radiation effect on polypropylene blends with styrene-butadiene-styrene co-polymers where they studied the generation and decay of free radicals using electron spin resonance for the establishment of kinetics involved in the process.
M. Hassan and his fellows [9] described the devulcanization of passenger car tire’s rubber thru mechanical-chemical process where they measured the sol content as well as get content of the devulcanized products. J. Shah and co-worker [10] studied the effect of Calcium carbide catalyst on distribution of pyrolysis product of rubber where they described that temperature increase in the presence of Calcium carbide catalyst increases liquid fraction as well as total evolved gases while decreasing char yield. They reported that their product liquid had high calorific value; GCV (42.8 MJ kg-1) with boiling point of 320oC. Their results indicated that viscosity, freezing point, specific gravity and diesel index of the liquid product were comparable to diesel fuel. A review presented by A. Quek and R. Bala Subramanian [11] reveals that pyrolytic oil products obtained from various types of waste tires as well as their pyrolysis operating conditions may yield useful chemicals such as Limonene, aromatic Benzene and a like compounds.
Similar findings were reported by G. Choi and co-workers [12] where they used a fixed bed reactor to pyrolyze waste tire rubber at temperature ranging for 500oC-800oC. They reported the presence of aromatic hydrocarbons, limonene along with some heteroatom-containing compounds like 2,4-dimethylquinoline and benzothiazole in their pyrolyzed oil products. G. I. Danmaliki and T. A. Saleh [13] investigated conversion of scrap tires into activated carbon where nitric acid was used to oxidize the functional groups on recycling for production of oil and gas form waste tire pyrolysis where they reported the upgradation of oil quality by increased construction of derived oil and gases such as H2 as well as CO during pyrolysis. A. H. Ahoor and N. Z. Atashbar [18] used MgCl2 catalyst in a batch reactor for pyrolysis of waste tire under inert atmosphere, where they optimized conditions to 407.3oC pyrolysis temperature, 12.5 mm particle size, 133.7 mL min 1 flow rate for 1800s pyrolysis time using 11.5wt% of catalyst. They reported that the product oil with physical properties such as cetane number, viscosity and density comparable to the commercial diesel fuel can be used as alternative fuel. Their results indicate that MgCl2 was helpful in reduction of the sulfur content of the final product. H. Aydin and co-workers [19] reported the use of Ca(OH)2 as catalyst for pyrolysis of waste tire rubber under nitrogen atmosphere, where they described the decrease in sulfur content of the product oil.
E. Yazdani, S. H. Hashemabadi, A. Taghizadeh [20] converted waste tire into a distillate mixture of light naphtha (14%), heavy naphtha (4%) and middle distillate (36%) in a rotating kiln reactor at temperature ranging from 400oC to 1050oC under inert atmosphere. Further literature reveals that waste tire rubber is pyrolyzed using a variety of catalysts including silica-alumina, mesoporous materials (MCM-type), clays (ZSM-5), zeolites and a like materials. Some synthetic zeolites were also used by different researchers but they are somehow expensive that affect the final cost for industry [21-24].In present study the use of economic and easily available MgO catalyst for the conversion of waste tube rubber at low temperature is being reported where its influence on the composition and yield of derived oil is investigated. The obtained product oils were investigated via various physical and chemical tests in order to ensure its potential use as fuel.
Materials and Methods
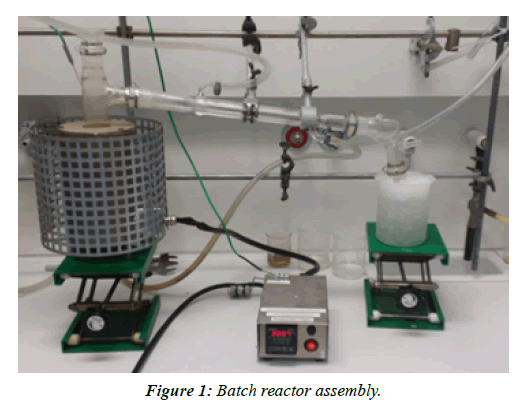
Batch reactor having 18 cm length and 3.3 cm width shown in Figure 1, was used to carry out pyrolysis experiments. A. R. Grade magnesium oxide catalyst was used for catalytic conversion of commercial waste tube rubber pieces (5 mm-8 mm cross-section width). Catalyst to tube rubber ratio was 1:5 for experiment. The reactor was heated from 250oC-350oC in an automatic programmed electric furnace, where temperature was controlled by a thermocouple. After on hour of heating, glass tube was removed from the reactor and was allowed to cool down to room temperature. The pyrolysis products obtained were identified as gas, liquid (oil) and char (solid). The weight of these products was measured accordingly and product composition was calculated via formula:
% Oil = (Wt. of oil ) / (Wt. of tube rubber )×100
% Residue = (Wt. of residue) / (Wt. of tube rubber )×100
% Gas = % Conversion − (% Oil gr % Residue)
The efficiency of the reaction was measured by percent conversion using the following formula:
% Conversion =(Wt. of tube rubber −Wt. of residue)
Treatment operations
Data about the treatment of municipal waste have to be reported according to a classification system of five treatment operations. For the purpose of the present paper, the five treatment operations are aggregated to the following three treatment classifications:
• Landfilling: “Landfill/disposal (D1-D7, D12)”
• Incineration: “Incineration for disposal (D10)” and “Incineration for energy recovery (R1)”
• Recycling: “Material recycling” and “Composting and digestion”
Due to losses (e.g. water and carbon dioxide losses during biological treatment) the sum of the treatment operations equals not always the quantity of waste generated. For the calculation of the rates of the separate treatment operations, the quantity of each treatment operation is related to the waste generated and not to the sum of the treatment operations. The reason is that the official calculation of the recycling and landfilling rates relates to waste generated.
Calculation method and assumptions
The recycling potential is estimated by calculating for each European Member State the difference between the existing recycling (2018/2017) and the recycling rates of the subsequent target year (2020) as well as between the recycling rates of two consecutive target years (2020-2025, 2025-2030, 2030-2035). The additional recycling quantity is compensated by reducing the landfilled waste. After the target for the landfilling rate of 10% is reached, incinerated waste quantities are reduced. If the landfilling rate of a Member State is already below 10%, it is not further reduced.
The data in the Eurostat database are used in the published way. No check of the correct application of definitions (e.g. recycling) and calculation rules is carried out. When calculating the recycling potential, it is assumed, that the Member States actually succeed in achieving requested target values. If a Member State reaches or over-achieves already the recycling rate of a specific target year, the reported data are not changed.
Regarding the Member States, which have the option to postpone the deadline for the recycling rate and/or the deadline for the landfilling rate; it is assumed that this option is actually used. In this case, it has to be taken into consideration that a landfilling rate of 25% has to be achieved for the year 2035.
Packaging Waste
Total packaging waste: The methodology for estimating the additional recycling potential regarding packaging waste is principally comparable to the methodology for municipal waste. Some differences, however, exist:
Data are extracted from the database “env_waspac” [20]. The reporting period is the year 2017, for which all necessary data are available for all Member States.
The data for packaging waste has to be reported according to five “waste management operations”. For the purpose of this calculation, these waste management operations are aggregated to the following two treatment classifications:
• Recycling: “Recycling-material” and “Recycling-other (including organic recycling, excluding material recycling)”
• Incineration/Other Recovery: This classification includes the three waste management operations “Recovery - energy recovery (R1)”, “Incineration with energy recovery (incineration at municipal waste incineration facilities that do not meet the energy efficiency criteria)” and “Other recovery”
The treatment classification does not add up to 100%, because no data about the disposal of packaging waste have to be reported. Therefore, the difference between packaging waste generated and packaging waste, which is recycled or otherwise recovered, is regarded as disposal.
Like for municipal waste, the additional recycling quantity is compensated by reducing the landfilled waste. As the Landfill Directive 2018/850 /EU (European Union, 2018b) does not contain a specific maximum target for landfilling of packaging waste, but the obligation not to landfill waste that was separately collected for recycling purposes, the landfilling of packaging waste is reduced to zero, before the incineration is reduced.
Individual packaging materials: The Packaging Waste Directive includes not only target values for the total packaging waste (whole group), but also for five packaging materials (within the group). The calculation of recycling potentials is also carried out for each packaging material, except for metals. The reason is that existing target values and reporting on recycling rates covers metal packaging as a whole, while the future provisions are specified for ferrous metals and aluminium separately. For the year 2017, already 10 Member States have voluntarily reported data on the two subcategories. These reports show no uniform proportion between the quantities of ferrous metals and of aluminium, which varies from approximately 10%/90% in France to 45%/55% in Sweden. Therefore, a consistent estimation of a recycling potential for metals is not possible.
Furthermore, 22 Member States have reported data on “other packaging”, which can comprise materials like textiles, ceramic or cork. For these materials, the Packaging Waste Directive does not prescribe target values for recycling rates. As reported quantities are small (about 253,000 tonnes for all 22 Member States), the recycling potential for this fraction is not calculated.
In contrast to municipal waste, the possibility for Member States to postpone the deadlines for certain packaging materials is not considered in the calculation of the recycling potential. The reason is, that there are too many options for the Member States to choose from and it cannot be estimated, which ones will be actually selected.
Results
Condition optimizations for the yield of catalytic cracking of tube rubber were performed in a systematic way. Physical and Chemical test of the oil products were performed.
Temperature Optimization for Catalytic Cracking of Tube Rubber
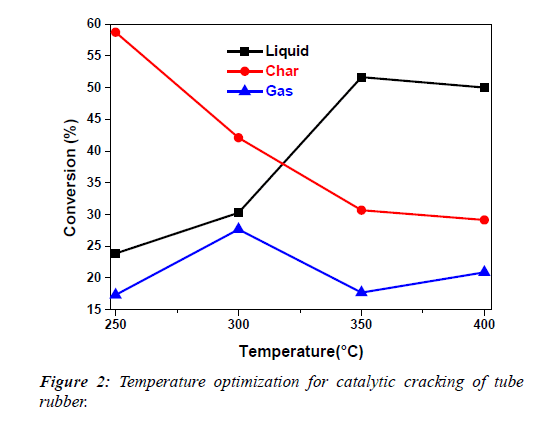
Temperature optimization results of tube rubber are shown Table 1 and Figure 2. It can be seen that increase in temperature from 250°C-350°C subsequently increases the amount of liquid while decreases char (solid). The total conversion of tube rubber is 99.6% at 350°C. This indicates the completion of reaction at 350°C. The maximum conversion of oil took place at 350°C and was selected as the optimum temperature for further work.
Table.1: Temperature optimization for catalytic cracking of tube rubber.
| S.No | Temp (°C) | % (L) ± S.D | % (S) ± S.D | % (G) ± S.D | T. Con ± S.D |
|---|---|---|---|---|---|
| 1 | 250 | 23.84 ± 1.38 | 58.73 ± 1.60 | 17.30 ± 1.34 | 99.88 ± 0.21 |
| 2 | 300 | 30.26 ± 0.14 | 42.10 ± 0.10 | 27.64 ± 0.04 | 100.0 ± 0.00 |
| 3 | 350 | 51.66 ± 0.56 | 30.66 ± 0.76 | 17.67 ± 0.50 | 99.60 ± 0.41 |
“Temp” stands for temperature, “L” for liquid “S” for solid, “G” for gas, “T. Con” for total conversion and “S.D” stands for standard deviation
Other authors also obtained results in the similar range but using high temperature of pyrolysis. They obtained maximum liquid yield (55 wt%) at a pyrolysis temperature of 550°C while the char yield was as low as 33 wt% and the gas yield was around 11 wt% [25].
Cunliffe and Williams [26] reported 58.2 wt% at 475oC which was adversely affected by the increase in temperature to 53.1 wt% at 600oC. Murillo and his fellows [27] reported an increased yield from 42.24 wt% to 60.02% at 425oC but a decrease to 54.1wt% at 500oC. Interestingly, they reported a decrease in char from 50.67 wt% to 26.41 wt% at 450oC and then remains constant. Que and his co-workers [28] reported 17.7% oil, 49.0% gas and 33.3% char from catalytic conversion waste tires at 430oC using ZSM-5 zeolite under inert atmosphere. Murugan et al. [29] performed pyrolysis of waste tires from 450°C to 650°C with heating rate of 5°C min-1 for 2. They reported 55% liquid fraction and 10% of gas as their products. Kar [30] investigated pyrolysis of used tires reporting 60.0 wt% maximum oil yield at 425oC, where it decreased to 54.12 wt% at 500oC with a decrease in char from 50.67 to 26.41 wt% and increase of gas from 2.99 wt% to 20.22 wt%. Literature reveals that increase in temperature consequently decreased the TPO yield, however 400°C-450oC in the optimum temperature range for increased tire pyrolysis oil yield [31].
The results confirm that with variations in temperature, changes not only occur in the per cent conversion but also in the nature of the products. It was expected that with increase in temperature polarity of the catalyst increase due to increase in vibrational energies of the catalyst. Thus, charge induction also increases resulting in faster reaction.
Weight of Catalyst Optimization for Catalytic Cracking of Tube Rubber
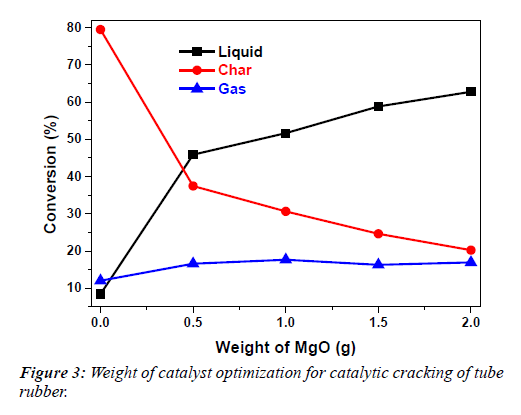
Table 2 and Figure 3 show that amount of liquid increases and that of solid decreases with increase of weight of catalyst.
Table 2: Weight of catalyst optimization for catalytic cracking of tube rubber.
| S. No | W(g) | % (L) ± S.D | % (S) ± S.D | % (G) ± S.D | T.Con ± S.D |
|---|---|---|---|---|---|
| 1 | 0 | 8.46 ± 0.18 | 79.46 ± 0.42 | 12.06 ± 0.589 | 100 ± 0.00 |
| 2 | 0.5 | 45.89 ± 6.37 | 37.48 ± 3.07 | 16.64 ± 3.32 | 100 ± 0.00 |
| 3 | 1 | 51.66 ± 0.56 | 30.6 ± 0.76 | 17.67 ± 0.50 | 99.66 ± 0.41 |
| 4 | 1.5 | 58.82 ± 2.71 | 24.8 ± 1.29 | 16.31 ± 2.04 | 100 ± 0.000 |
| 5 | 2 | 62.77 ± 1.76 | 20.26 ± 0.64 | 16.96 ± 1.13 | 100 ± 0.000 |
“W” stands for weight of catalyst, “L” for liquid “S” for solid, “G” for gas, “T.Con” for total conversion and “S.D” stands for standard deviation
The release of gases is almost constant with 0.5 g-2.0 g of catalyst. 2 g of catalyst was selected as optimum weight for cracking of butyl rubber because it produces approximately the same amount of char (carbon black) as present in the formulation of butyl inner tube [1,2].
Heating Time and Rate Optimization for Catalytic Cracking of Tube Rubber
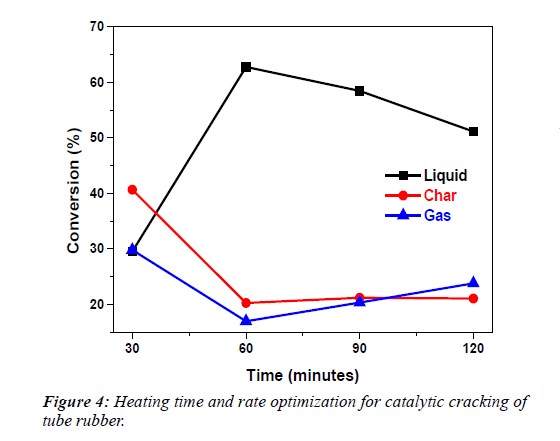
It can be seen from the Table 3 and Figure 4, that the rate of reaction increases with heating time.
Table 3: Heating time and rate optimization for catalytic cracking of tube rubber.
| S.No | Time (min) | %(L) ± S.D | %(S) ± S.D | %(G) ± S.D | %T.Con+S.D |
|---|---|---|---|---|---|
| 1 | 30 | 29.53 ± 0.44 | 40.66 ± 0.31 | 29.80 ± 0.14 | 100.0 ± 0.00 |
| 2 | 60 | 62.76 ± 1.75 | 20.26 ± 0.64 | 16.96 ± 1.13 | 99.99 ± 0.01 |
| 3 | 90 | 58.46 ± 1.58 | 21.20 ± 0.53 | 20.30 ± 2.09 | 99.99 ± 0.01 |
| 4 | 120 | 55.13 ± 0.69 | 21.06 ± 0.50 | 23.80 ± 1.17 | 100.0 ± 0.00 |
Where,“min” stands for heating time, “L” for liquid “S” for solid, “G” for gas, “T.Con” for total conversion and “S.D” stands for standard deviation.
Maximum formation of oil (liquid) took place when heating time was 60 min. The amount of char after 60 minutes heating remains constant but the amount of gases increases due further cracking of oil at longer time of cracking. Therefore, 60 min was selected as optimum time for heating for further investigations. By using MgO as catalyst two types of fractions i.e. Light oil and Heavy oil fractions were obtained, which were analyzed physically shown in Table 4; The density of the light oil fraction is similar to the density of diesel fuel and the distillation data is also similar to the distillation data of diesel fuel in the range of 180°C-340°C. The distillation data from 20°C-100°C is also in the range of kerosene distillate (165°C-230°C) and 40% of distillate is in the range of aromatic kerosene (200°C-265°C). Viscosity and specific gravity is in the range of aromatic kerosene.
Table 4: Analysis of light oil and heavy oil fractions.
| Parameters | Light Oil Values | Heavy Oil Values |
|---|---|---|
| Density (g/ml) | 0.8432 | 0.9992 |
| Specific Gravity | 0.8432 | 0.9992 |
| Viscosity (centipoises) | 1.63 | 1.046 |
| API Gravity | 36.31 | 10.11 |
| Kinematic (mm2/sec) | 1.933 | 1.0468 |
| Aniline Point (°C) | 69 | 52 |
| Flash Point (°C) | 39 | 45 |
| Diesel Index | 56.71 | 12.69 |
| Kw Valve | 9.255 | 6.695 |
Density of heavy oil fraction is near to density of heavy fuel oil and flash point is near to kerosene oil. The distillation data shows 80% of light oil, 10% of light naphtha and 10% naphthalene oil. 30% of oil fraction in the range of 158°C-200°C also shows the presence of phenol oil, which was also confirmed by phenol determination.
Fuel tests shows that both light oil and heavy oil fractions are complex mixtures of hydrocarbons.
Oil obtained was analyzed chemically. From Bromine Water Test, the solution got faint colour immediately which indicates the presence of olefins in oil. In Potassium Permanganate Test, the solution lost its color immediately which indicates the presence of olefins as a major component in oil. From calibration plot, the concentration of phenol was determined in each sample shown in Figure 5. The amount of phenols found in heavy oil fraction is 90μg/100ml, which was also confirmed by the distillation fraction of heavy oil in the range of 158°C-200°C. The distillation temperature range for phenolic oil is (167°C-194°C).
Bromine Number determined for heavy oil fraction is 70 g/100 g which indicates the presence of high concentration of olefinic compounds.
Discussion and Conclusions
Used tube rubber can advantageously be recycled via catalytic pyrolysis method. It has been revealed that conversion of tube rubber in presence of MgO catalyst at lower temperature into valuable liquid is a practicable procedure. Oil obtained from catalytic conversion of tube rubber at optimized conditions has shown resemblance to conventional fuel both in physical as well as its chemical properties. Catalytic pyrolysis of tube rubber is a useful recycling process both from economic as well as environmental view point. However, more in-depth studies are required to investigate these liquid fractions separately. Furthermore, char obtained during the catalytic process may be a source of activated carbon that can usefully be utilized in various process such as wastewater treatment. Here it can be concluded that oils obtained from catalytic conversion of waste tube rubber might be developed as alternative to conventional mineral oil.
References
- https://www.uspto.gov/patents/patents-announcements/uspto-statement-ceasing-annual-top-10-patents-holders-list
- Lin JP, Chang CY, Wu CH. Pyrolytic treatment of rubber waste: Pyrolysis kinetics of styrene—butadiene rubber. J Chem Tech Biotech. 1996;66:7-14.
- Miranda M, Pinto F, Gulyurtlu I. Pyrolysis of rubber tyre wastes: A kinetic study. Fuel. 2013;103:542-52.
- Raj RE, Kennedy ZR, Pillai BC. Optimization of process parameters in flash pyrolysis of waste tyres to liquid and gaseous fuel in a fluidized bed reactor. Energy Convers Manag. 2013;67:145-51.
- Telnov AV, Zavyalov NV, Khokhlov YA. Radiation degradation of spent butyl rubbers. Radiat Phys Chem. 2002;63:245-48.
- ?en, M, Uzun C, Kanto?lu O. Effect of gamma irradiation conditions on the radiation-induced degradation of isobutylene–isoprene rubber. Nucl Instrum Methods Phys Res B. 2003;208:480-84.
- Perera R, Albano C, Gonzalez J, et al. The effect of gamma radiation on the properties of polypropylene blends with styrene–butadiene–styrene copolymers Polym Degrad Stab. 2004;85:741-50.
- Hassan MM, Badway NA, Elnaggar MY, et al. Thermo-mechanical properties of devulcanized rubber/high crystalline polypropylene blends modified by ionizing radiation. J Ind Eng Chem. 2013;19:1241-50.
- Shah J, Jan MR, Mabood F. Catalytic conversion of waste tyres into valuable hydrocarbons. J Polym Environ. 2007;15:207-11.
- Quek A, Balasubramanian R. Liquefaction of waste tires by pyrolysis for oil and chemicals-A review. J Anal Appl Pyrolysis. 2013;101:1-6.
- Choi GG, Jung SH, Oh SJ, et al. Total utilization of waste tire rubber through pyrolysis to obtain oils and CO2 activation of pyrolysis char. Fuel Process Technol. 2014;123:57-64.
- Danmaliki GI, Saleh TA. Influence of conversion parameters of waste tires to activated carbon on adsorption of dibenzothiophene from model fuels. J Clean Prod. 2016;117:50-55.
- Yu BC, Jung JW, Park K, Goodenough JB. A new approach for recycling waste rubber products in Li–S batteries. Energy Environ Sci. 2017;10:86-90.
- Czajczynska D, Krzy?y?ska R, Jouhara H, Spencer N. Use of pyrolytic gas from waste tire as a fuel: A review. Energy. 2017;134:1121-31.
- Seljak T, Oprešnik SR, Katrašnik T. Combustion characteristics of tire pyrolysis oil in turbo charged compression ignition engine. Fuel. 2015;150:226-35.
- Nagano H. Exxon butyl rubber compounding and applications. Yokohama: Exxon Chemical Japan Polymers Technical Center.
- Luo S, Feng Y. The production of fuel oil and combustible gas by catalytic pyrolysis of waste tire using waste heat of blast-furnace slag. Energy Convers Manag. 2017;136:27-35.
- Ahoor AH, Zandi-Atashbar N. Fuel production based on catalytic pyrolysis of waste tires as an optimized model. Energy Convers Manag. 2014;87:653-69.
- Ilkilic C, Ayd?n H. Fuel production from waste vehicle tires by catalytic pyrolysis and its application in a diesel engine. Fuel Processing Tech. 2011;92:1129-35.
- Yazdani E, Hashemabadi SH, Taghizadeh A. Study of waste tire pyrolysis in a rotary kiln reactor in a wide range of pyrolysis temperature. Waste Manag. 2019;85:195-01.
- Shah J, Jan MR, Mabood F, Conversion of waste tyres into carbon black and their utilization as adsorbent. J Chin Chem Soc. 2006;53:1085-89.
- Shah J, Jan MR, Mabood F. Catalytic conversion of waste tyres into valuable hydrocarbons. J Polym Environ 2007;15:207-11
- Shah J, Rasul Jan M, Mabood F. Catalytic pyrolysis of waste tyre rubber into hydrocarbons via base catalysts. Iran J Chem Chem Eng. 2008;27:103-09.
- Mabood F, Jan MR, Shah J. Catalytic conversion of waste inner tube rubber (isobutylene isoprene) into valuable products. J Chem Soc Pak. 2010;32:767-73
- Sui H, Wang X, Wu Z. Influence of Temperature and Residence Time of Gas on Property of Waste Tyre Pyrolysis. J Biobased Mater Bioenergy. 2020;14:98-107.
- Cunliffe AM, Williams PT. Composition of oils derived from the batch pyrolysis of tyres. J Anal Appl Pyrolysis. 1998;44:131-52.
- Murillo R, Aylón E, Navarro MV. The application of thermal processes to valorise waste tyre. Fuel processing tech. 2006;8:143-47.
- Murillo R, Aylón E, Navarro MV. The application of thermal processes to valorise waste tyre. Fuel processing tech. 2006;87:143-47.
- Murugan S, Ramaswamy MC, Nagarajan G. The use of tyre pyrolysis oil in diesel engines. Waste manag. 2008;28:2743-49.
- Kar Y. Catalytic pyrolysis of car tire waste using expanded perlite. Waste Manag. 2011;31:1772-82.
- Sathiskumar C, Karthikeyan S. Recycling of waste tires and its energy storage application of by-products–a review. Sustain Mater Techn 2019;22:e00125.