Case Report - Current Pediatric Research (2019) Volume 23, Issue 2
Complete androgen insensitivity syndrome in a Saudi adolescent Girl: A case report
Afnan A Bahamim1, Noor S Bawahab2, Abdulmoein E Al-Agha3*
1Faculty of Medicine Ibn Sina National College for Medical Studies, Jeddah, Saudi Arabia
2Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
3Department of Pediatrics, King Abdulaziz University, Jeddah, Saudi Arabia
- *Corresponding Author:
- Abdulmoein Eid Al-Agha
Professor of Pediatric Endocrinology Head
Pediatric Endocrinology & Diabetes section
King Abdulaziz University Hospital
Pediatric Department
P.O. Box 80215, Jeddah 21589, Saudi Arabia
Tel: 00201001097818
Fax: + 966 2 640 3841, + 966 2 6408353
E-mail: aagha@kau.edu.sa
Accepted on April 24th, 2019
Abstract
Complete androgen insensitivity syndrome (CAIS) is a rare X-linked disorder, resulting in impaired development of male physical traits. Human genital virilization occurs between 8 and 14 weeks of gestation and is compromised by defects in androgen secretion or functioning androgen receptors. CAIS occurs in an estimated 1 in 20,000 to 64,000 newborn males. We report the case of a 16-year-old female with an XY karyotype, presenting with primary amenorrhea and external female phenotype genitalia. Magnetic resonance imaging confirmed the absence of female internal genital organs and the presence of bilateral inguinal testes. Serum testosterone levels were within the normal range for males. The patient was diagnosed with CAIS. Many more cases need to be studied for a better understanding and awareness of the condition. Prenatal screening for ambiguous genitalia and imaging for suspected cases at birth are critical to address future physiological and psychiatric issues.
Keywords
Primary amenorrhea, Complete androgen insensitivity syndrome, Androgen secretion.
Introduction
Androgen insensitivity syndrome (AIS) is a Mendelian mode of inheritance (X-linked) disorder with an estimated incidence of 1 in 20,000 to 1 in 62,000 males [1]. It is the third leading cause of primary amenorrhea during puberty, after gonadal dysgenesis, and the congenital absence of the vagina [2], and is characterized by androgen resistance that leads to impaired development of male physical traits [3].
Male foetal development depends on androgens for differentiation. Following normal development of the testes, the Leydig cells produce testosterone, which causes differentiation of the Wolffian duct into the epididymis, vas deferens, and seminal vesicles [4]. Conversion of testosterone to dihydrotestosterone by the enzyme 5α-reductase type 2 aids in the differentiation of male external genitalia [5]. In humans, the crucial period for genital virilization is between 8 and 14 weeks of gestation and depends on androgen secretion and functioning androgen receptors [6]. Any defects in either will compromise the virilization process. AIS presents with a broad spectrum of defects in the external genitalia, depending on the degree of androgen responsive-ness. The defects can be subdivided into three phenotypes: complete androgen insensitivity syndrome (CAIS) with typical female external genitalia, partial androgen insensitivity syndrome (PAIS) with predominantly male or ambiguous external genitalia, and mild androgen insensitivity syndrome (MAIS) with typical male external genitalia or an isolated micropenis but with gynecomastia at puberty and infertility in adulthood [7].
CAIS is a classic type of AIS with an estimated prevalence of 1 in 20,000 to 64,000 newborn males [8]. Clinical diagnosis of CAIS is established following the typical presentation of primary amenorrhea at puberty or an inguinal hernia and labial swelling in a female infant with a 46, XY karyotype [9]. These infants are nearly always raised and identified as females and are considered to be anatomically and legally female. The reason for this is the complete absence of any sign of masculinization in the external genitalia [10]. We report the case of a 16-year-old female who presented with primary amenorrhea and was found to have an XY karyotype. On further investigation, she was diagnosed with CAIS.
Case Presentation
A 16-year-old Saudi female presented to the paediatrics endocrinology clinic for the evaluation of primary amenorrhea. She was born of a spontaneous vaginal delivery following a full-term uneventful pregnancy. Her birth weight was 3 kg and length was 50 cm. The external genitalia exhibited the female phenotype at birth. There was no history of an inguinal mass or inguinal hernia repair. She was born of second-degree consanguineous parents and her older sister had also presented with a similar history and complaint. On examination, her weight was 53 kg (in the 50th percentile) and height was 163 cm (in the 90th percentile), with a complete female phenotype. Her breast development and axillary and pubic hair growth were at Tanner stage 2. There was no facial or body hair, dysmorphic features, or deep voice. Genital examination revealed bi-lateral inguinal swellings with palpable gonads and normal external genitalia. Other systemic examination findings were unremarkable. She underwent laboratory investigations as shown in Table 1.
| Lab Test | Results | Reference Range |
|---|---|---|
| White Blood Cell Count (WBC) | 5.79 K/μl | 4.5-11.5 K/μl |
| Red Blood Cell Count (RBC) | 5.72 M/μl | 4.04- 5.48 M/μl |
| Haemoglobin | 15.3 g/dl | 12.0-15.0 g/dl |
| Haematocrit | 44.40% | 35-49% |
| Mean Cell Volume (MCV) | 77.6 fl | 80-94 fl |
| Mean Cell Haemoglobin (MCH) | 26.7 pg | 32-36 pg |
| MCHC | 34.50% | 32-36% |
| RDW-CV | 13.4 | - |
| Platelets | 404 K/μl | 150-450 K/μl |
| Haemoglobin A1C (HbA1c) | 8.10% | 4.2-6.3% |
| TSH (Thyroid Stimulating Hormone)-TFT | 2.80 μlU/L | 0.27-4.2 μl U/L |
| Free T4 Hormone (FT4)-TFT | 15.4 pmol/L | 12-22 pmol/L |
| Luteinizing Hormone (LH) | 6.2 mlU/L | Male: 0.8-6.1 mlU/L |
| Oestradiol Hormone | 51 pmol/L | 26-125 pmol/L |
| Follicle Stimulating Hormone (FSH) | 10.7 lU/L | - |
| Testosterone Hormone | 15 nmol/L | male 8.4-28.7 nmol/L |
| Female 0.5 – 2.6 nmol/L | ||
| Phosphate (Phos) | 1.24 mmol/L | 0.8-1.58 mmol/L |
| Calcium | 2.37 mmol/L | 2.12-2.52 mmol/L |
| Vitamin D total (25-OH-Vitamin D3) | 49.0 nmol/L | 75-250 nmol/L |
| Bone mineral density (BMD) | Z-score of -2.7 | Z-score of -1 and above |
Table 1. Laboratory test results of the patient.
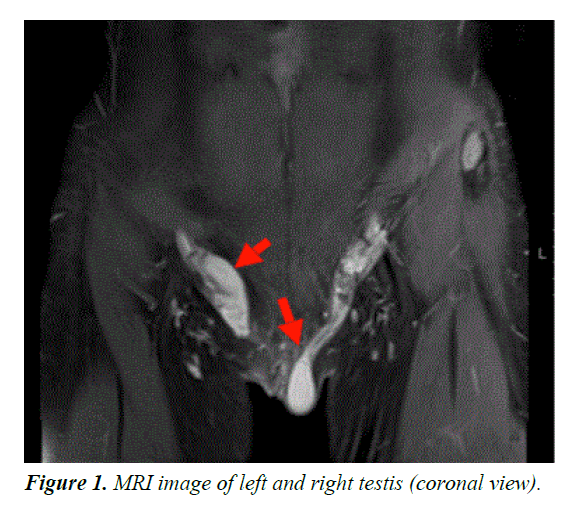
On magnetic resonance imaging, (MRI) well-defined, rounded, intermediate-to-high signal intensity structures were noted in the inguinal regions, bilaterally. The structure in the left groin measured 2.2 × 1.6 × 3 cm in anteroposterior, transverse, and craniocaudal dimensions, respectively. The structure in the right inguinal region measured 1.8 × 2.5 × 3.6 cm. No female reproductive organs were identified. The external genitalia were otherwise difficult to clearly characterize, and a definite prostate was not noted. The other pelvic organs were unremarkable.
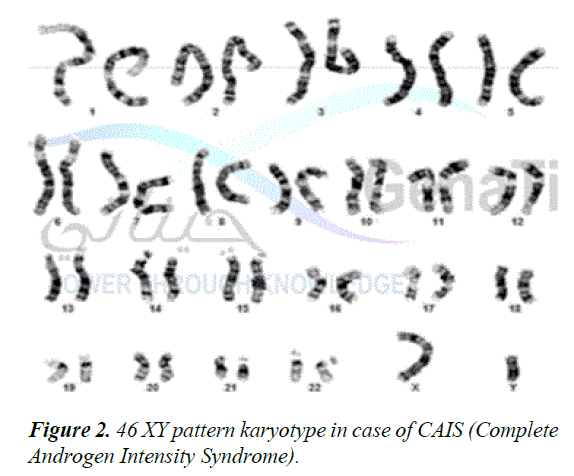
MRI confirmed the absence of female internal genital organs, such as the uterus, fallopian tubes, or ovaries and demonstrated the presence of bilateral inguinal testes as shown in Figure 1. Moreover, karyotyping revealed a male genotype (46, XY) (Figure 2). A diagnosis of CAIS was established based on the karyotype. The patient was started on oestrogen replacement therapy and was referred to the paediatric surgeon for bilateral orchidectomy.
Discussion
CAIS accounts for approximately 10% of primary amenorrhea cases [2]. To our knowledge, this is the first case reported in a Saudi girl. The first known AIS case was reported in 1953 by JM Morris, an American gynaecologist [11]. In one documented report, an adolescent female presented with primary amenorrhea, breast development, pubertal growth, and absent or sparse growth of pubic and axillary hair [1]. However, 1-2% of the cases present in the neonatal period with the appearance of bilateral inguinal or labial swellings; Kim et al. reported a 3-year-old girl diagnosed during hernia repair [12,13]. Our patient did not present with an inguinal mass or undergo any hernia repair. According to a published study, in 50–70% of cases, the position of the testes is intra-abdominal [1]. Intra-abdominal testes are at risk for malignant transformation. Fortunately, since the testes are normal in CAIS, tumour development is rare in puberty, with an incidence of 0.8% [14]; in adults the risk increases to 14% (range 0-22%) [15]. Therefore, laparoscopic gonadectomy is recommended soon after puberty, although the timing of the surgical procedure is controversial [9,15]. Our patient was referred to the paediatric surgeon for bilateral orchidectomy as soon as possible.
Patients with CAIS typically present with a blind-ending vagina. However, in 77% of the patients the vagina is small and tight, and 35% present with vaginal hypoplasia, which usually requires surgical reconstruction [16]. In our patient, examination revealed a narrow vaginal opening.
In addition, CAIS patients tend to have low bone mineral density (BMD), which can be managed by hormone replacement therapy with adjuvant calcium and vitamin D supplements [17]. Our patient had a BMD Z-score of -2.7 and a total Vitamin D level of 49.0 nmol/l. Her levels of luteinizing hormone and serum estradiol were higher than those in males, but lower than those in females without CAIS, and serum testosterone levels were within or above the normal range for males [18,19]. Further, in 2000, a cohort study concluded that the average height in a female with CAIS was 174 cm, 7.2% taller than the average height of an adult American female (162.3 cm) [20]. Our patient was 163 cm tall, a 5.2% increase compared with the height of same-age adolescent Saudi girls.
Conclusion
In summary, bilateral undescended testis in imaging and a testosterone level in the normal range for males were the major findings of our case. CAIS is a rare disorder and many more cases need to be studied to acquire a better understanding and awareness of the disease. Prenatal screening for ambiguous genitalia should be done as early as possible, followed by imaging of any suspected cases at birth to address future physical and psychiatric issues.
We present a case of infant CdLS with generalized hypertrichosis that we consider of interest to increase pediatrician awareness of the differentiation between hypertrichosis and hirsutism, which are two different types of excessive hair growth. Moreover, pediatricians should be aware of the possible causes of generalized hypertrichosis.
References
- Bhaskararao G, Himabindu Y, Nayak SR, et al. Laparoscopic gonedectomy in a case of complete androgen insensitivity syndrome. J Hum Reprod Sci. 2014; 7: 221-223.
- Sharma S, Balwan WK, Kumar P, et al. Androgen insensitivity syndrome (testicular feminization). J Ousted Gingerol India. 2012; 62: 199-201.
- Souhail R, Amine S, Nadia A, et al. Complete androgen insensitivity syndrome or testicular feminization: review of literature based on a case report. Pan Afr Med J. 2016; 25: 199.
- Imperato-McGinley J, Zhu YS. Androgens and male physiology the syndrome of 5alpha-reductase-2 deficiency. Mol Cell Endocrinol. 2002; 198: 51-59.
- Mendonca BB, Batista RL, Domenice S, et al. Steroid 5α-reductase 2 deficiency. J Steroid Biochem Mol Biol. 2016; 163: 206-211.
- Mendonca BB, Domenico S, Arnhold IJ, et al. 46, XY disorders of sex development (DSD). Clin Endocrinol (Oxf). 2009; 70: 173-187.
- Mongan NP, Tadokoro-Cuccaro R, Bunch T, et al. Androgen insensitivity syndrome. Best Pract Res Clin Endocrinol Metab. 2015; 29: 569-580.
- Mendoza N, Motos MA. Androgen insensitivity syndrome. Gynecol Endocrinol. 2013; 29: 1-5.
- Hughes IA, Davies JD, Bunch TI, et al. Androgen insensitivity syndrome. Lancet. 2012; 380: 1419-1428.
- Gulia C, Baldassarra S, Zangari A, et al. Androgen insensitivity syndrome. Eur Rev Med Pharmacol Sci. 2018; 22: 3873-3887.
- Morris JM. The syndrome of testicular feminization in male pseudohermaphrodites. Am J Obstet Gynecol. 1953; 65: 1192-1211.
- Kim ES, Warner BW. Unexpected finding during inguinal hernia repair in a girl. Surgery. 2005; 138: 954-955.
- Hurme T, Lahdes-Vasama T, Makela E, et al. Clinical findings in prepubertal girls with inguinal hernia with special reference to the diagnosis of androgen insensitivity syndrome. Scand J Urol Nephrol. 2009; 43: 42-46.
- Rajpert-DeMeyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: Genetic and environmental aspects. Hum Reprod Update. 2006; 12: 303-323.
- Deans R, Creighton SM, Liao LM, et al. Timing of gonadectomy in adult women with complete androgen insensitivity syndrome (CAIS): Patient preferences and clinical evidence. Clin Endocrinol (Oxf). 2012; 76: 894-898.
- Minto CL, Liao KL, Conway GS, et al. Sexual function in women with complete androgen insensitivity syndrome. Fertil Steril. 2003; 80: 157-164.
- Sobel V, Schwartz B, Zhu YS, et al. Bone mineral density in the complete androgen insensitivity and 5α-reductase-2 deficiency syndromes. J Clin Endocrinol Metab. 2006; 91: 3017-3023.
- Melo KY, Mendonca BB, Billerbeck AE, et al. Clinical, hormonal, behavioral, and genetic characteristics of androgen insensitivity syndrome in a Brazilian cohort: Five novel mutations in the androgen receptor gene. J Clin Endocrinol Metab. 2003; 88: 3241-3250.
- Hughes IA, Deeb A. Androgen resistance. Best Pract Res Clin Endocrinol Metab. 2006; 20: 577-598.
- Marcus R, Leary D, Schneider DL, et al. The contribution of testosterone to skeletal development and maintenance: lessons from the androgen insensitivity syndrome. J Clin Endocrinol Metab. 2000; 85: 1032-1037.