- Biomedical Research (2014) Volume 25, Issue 3
Comparison of the diagnostic values in rheumatoid arthritis: Anti-CCP antibodies and other serological tests.
Doğan M1*, Küçüksaraç S2, Tüfekçi O3, Feyzioğlu B1, Özdemir M1, Baykan M1, Baysal B11Medical Microbiology Department, Necmettin Erbakan University, Meram Medical School, Konya/Turkey
2Physical Medicine and Rehabilitation Clinic, Beyhekim State Hospital, Konya/Turkey
3Physical Medicine and Rehabilitation Clinic, Farabi Private Hospital, Konya/Turkey
- *Corresponding Author:
- Metin Doğan
Department of Medical Microbiology
Necmettin Erbakan University
Meram Faculty of Medicine
Meram, Konya/Turkey 42080
Accpted April 25 2014
Abstract
Rheumatoid arthritis (RA) is an inflammatory autoimmune disease. Early diagnosis, early and aggressive treatment are the best means of avoiding joint destruction, organ damage and disability. This prospective study was carried out to determine the relationship between RA and laboratory tests used in the diagnosis of RA. 199 patients (20 males, 179 females; mean age: 50.6 years) that were suspected to be RA were included in the study. Anti-CCP, RF, ESR, and CRP tests were performed for all patients. The patients were divided two groups named RA and non-RA according to American College of Rheumatology criteria. Of the all patients, 96(48.2%) tested as positive for anti-CCP antibodies. Of the RA patients, 93 (68.9%) were positive for anti-CCP antibodies. The positivity of anti-CCP antibodies was 4.7% (3/64) for non-RA. Sensitivity, specificity, PPV, and NPV in diagnosis of RA for anti- CCP respectively were 0.69, 0.95, 0.97, and 0.59, and for high positive anti-CCP respectively were 0.55, 1.00, 1.00, and 0.52, and for RF respectively were 0.59, 0.92, 0.94, and 0.52, and for high positive RF respectively were 0.54, 0.98, 0.99, and 0.54, and for CRP respectively were 0.73, 0.78, 0.87, and 0.57, and for ESR respectively were 0.84, 0.30, 0.72, and 0.46. Sensitivity for the diagnosis of RA could be further increased by a combination of the anti-CCP and RF tests. There was a positive correlation between anti-CCP and RF positivity (?=0.59537), anti-CCP and RF high positivity (?=0.537364), ESR and CRP (?=0.426544). It is concluded that performing anti-CCP test in the diagnosis of RA could be beneficial since it has high specificity and positive predictive value. If this test was performed together with RF, it may be more beneficial.
Keywords
Anti-cyclic citrullinated peptide; rheumatoid factor; ESR; CRP; rheumatoid arthritis.
Introduction
Rheumatoid arthritis (RA) is an inflammatory autoimmune disease that affects joints primarily leading pain, stiffness and difficulty in movements. Cartilages, synovial cells, and some systems of the body are affected with autoimmunity and inflammatory processes of RA [1-4]. Early diagnosis and aggressive treatment are the best means of avoiding joint destruction, organ damage and disability [2,3,5,6].
Most of the chronic diseases have a gold standard tests for diagnosis. There is no such similar standard laboratory test for the diagnosis of RA. The classification criteria to define RA that is used internationally was defined by the American College of Rheumatology (ACR) in 1987 [7].
New criteria for rheumatoid arthritis classification were introduced in 2010 [8].
Citrulline-binding autoantibodies in RA patients was discovered by Nienhuis et al. in 1964, as an autoantibody able to bind to perinuclear granules in normal human buccal mucosa cells, and was named antiperinuclear factor [9]. In 1979, Young et al. reported that RA sera contained antibodies that reacted to the keratinized layer of epithelial cell.
These were called antikeratin antibodies, and were only found in RA patients [10]. High diagnostic specificity and sensitivity for first anti-CCP test associated with these antibodies were revealed by Boekel et al. in 2002 [11]. Anti- CCP2 test which demonstrates a higher sensitivity for RA was developed by Van Venrooij et al [12].
Anti-CCP was included in the ACR/EULAR (European League Against Rheumatism) RA classification criteria in 2010. Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and rheumatoid factor (RF) are the other serological tests used in the RA classification criteria [8].
This prospective study was carried out to determine the relationship between RA and laboratory tests used in the diagnosis of RA.
Materials and Methods
This study was carried out in patients clinically considered to be RA who were admitted at the physical medicine and rehabilitation (PMR) clinics of Meram Medical School Hospital and Konya Training and Research Hospital between January 2008 and December 2010 prospectively. The study protocol was approved by the Ethical Committee of Meram Medical School.
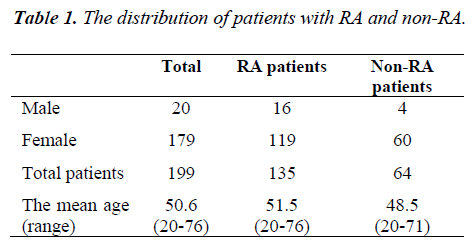
A total of 199 patients which consist of 20 males and 179 females were between 20 to 76 years old; who were suspected to be RA were included in the study (mean age: 50.6 years). All patients were examined, and radiologic and serological tests were performed. Clinical and other findings were assessed according to 1987 ACR criteria until September 2010 and the 2010 ACR/EULAR criteria after September 2010. The patients were grouped into two groups (RA group, non- RA group) according to these criteria. The distribution of patients with RA and non-RA were demonstrated in Table I.
Anti-CCP, RF, ESR, and CRP tests were performed for all patients. For ESR testing, blood was drawn in BD Vacutainer glass tubes, and ESR was measured by using BD Seditainer (BD Diagnostics, United Kingdom). CRP and RF tests were performed using a nephelometry (Dade-Behring, Marburg, Germany). Anti-CCP2 antibodies were tested using Microparticle Enzyme Immunoassay (MEIA) (AxSYM; Abbott dignostic, Germany) in accordance with the manufacturer’s protocol.
Statistical analysis was made by the Statistical Package for the Social Sciences (SPSS Inc., Chicago, Illinois, USA, version 17.0) for Windows software program. The relationship between the RA diagnosis and laboratory serological tests, the correlation of RF/Anti-CCP and ESR/CRP was investigated by analyzing the phi correlation coefficient.
Results
During the study, 199 patients who had rheumatologic complaints and were suspected to be RA, were evaluated by using ACR criteria in 1987 and ACR/EULAR criteria in 2010. The mean age of these patients was 50.6 yrs. 179 (89.9%) of patient were female and 20 (10.1%) were male. 135 (67.8%) of the patients were diagnosed RA, and 64 (32.2%) patients were non-RA according to these criteria. Clinical signs and laboratory results of all patients were re-evaluated according to the 2010 ACR/EULAR criteria after September 2010 and all patients diagnosed RA were confirmed as RA while the others were confirmed as non-RA.
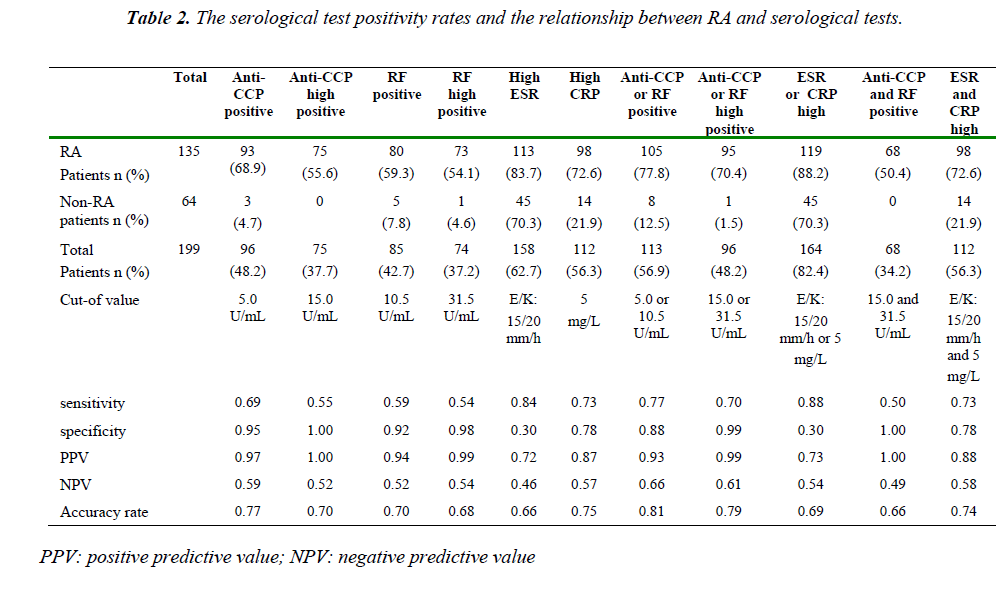
Ninety-six samples (48.2%) were tested as positive for anti-CCP antibodies in total of RA and non-RA patients. Of the RA patients, 93 (68.9%) were positive for anti-CCP antibodies. The positivity of anti-CCP antibodies was 4.7% (3/64) for non-RA. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) in diagnosis of RA for anti-CCP respectively were 0.69, 0.95, 0.97, and 0.59, and for high positive anti-CCP respectively were 0.55, 1.00, 1.00, and 0.52, and for RF respectively were 0.59, 0.92, 0.94, and 0.52, and for high positive RF respectively were 0.54, 0.98, 0.99, and 0.54, and for CRP respectively were 0.73, 0.78, 0.87, and 0.57, and for ESR respectively were 0.84, 0.30, 0.72, and 0.46. The serological test positivity rates and the relationship between RA and serological tests are shown in Table II. Anti-CCP and RF were respectively low positive in 18 and 7 of 135 RA patients, and in 3 and 4 of non-RA patients.
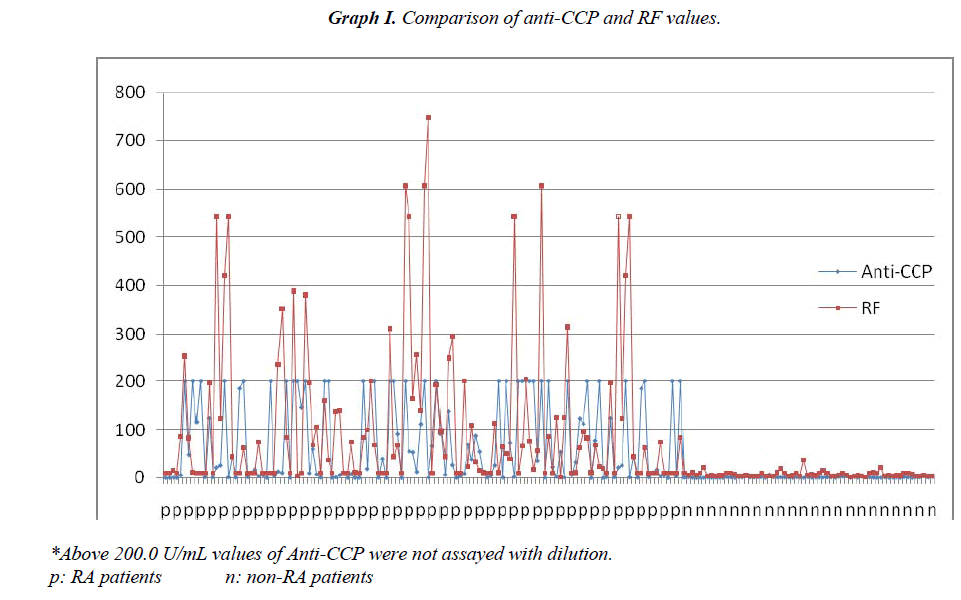
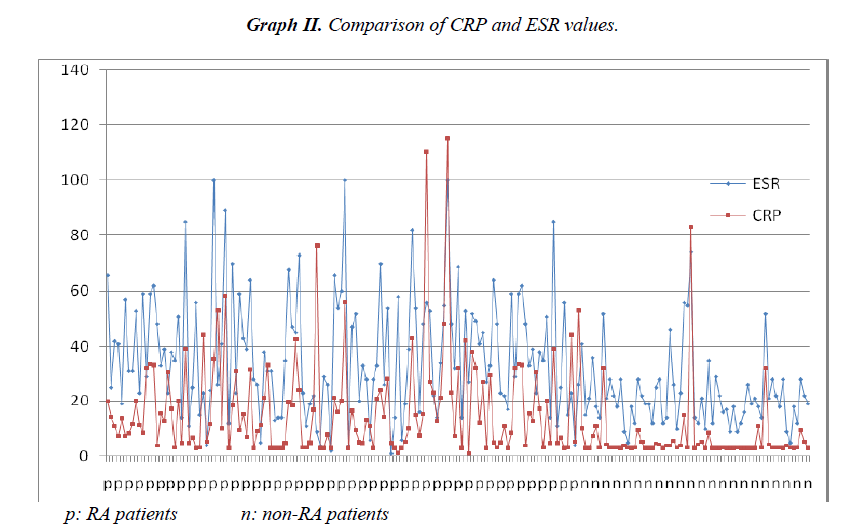
Sensitivity for the diagnosis of RA could be further increased by a combination of the anti-CCP and RF tests (Table II). There was a positive correlation between anti-CCP and RF positivity (Φ=0.59537), anti- CCP and RF high positivity (Φ=0.537364), ESR and CRP (Φ=0.426544). Comparison of anti-CCP and RF values for all patients are shown at Graphic I. Comparison of CRP and ESR values for all patients are shown at Graphic II.
Disclosures: None.
Discussion
Detection of IgM RF in serum was the only recommended laboratory marker within the 1987 RA diagnostic criteria of ACR [7]. Assessment of anti-CCP, RF, ESR and CRP in the serum was included in the ACR/EULAR RA classification criteria in 2010 [8]. Several studies have questioned the importance of anti-CCP antibody testing in distinguishing RA from other inflammatory diseases [13]. A standardized diagnostic evaluation study was performed on 524 RA patients with newly referred early arthritis. The strongest association between persistent or/and erosive arthritis and anti-CCP positivity was found [14]. Anti-CCP assays were done on sera from 242 RA patients who were followed up regularly for 3 years by Kastbom et al [15]. There was a positive correlation between anti-CCP and higher ESR, CRP. Also there was a positive correlation between RF and increased ESR and CRP [15]. Anti-CCP and RF positivities were closely associated in a study [16]. In this study fifty percent of the patients were both anti-CCP and RF positive. Anti-CCP was positive in sera from 208 of 379 patients (55%), and RF in sera from 235 of 374 patients (63%) [16]. Anti- CCP was found positive in 71/120 (59%) in RA patients’ sera by Koivula et al. [17]. Kim et al. performed a study to determine of seropositivity for RF and anti-CCP in unaffected first-degree relatives of RA patients (FDR) and RA patients. Seropositivity for RF and anti-CCP respectively was detected in 14.4% and 5.0% in FDR patients, and was detected 97,8% and 98,5% for RA patients and revealed significant positive correlations between RF and anti-CCP [18]. The anti-CCP was found positive in 68.7% of RA patients, and was found positive in 30.4% of RF negative patients in the performed study by Deveci et al.
They observed a significant difference in average RF levels when the anti-CCP positive and negative patients were compared; however, there was no significant difference between other parameters [19].
In a study, anti- CCP were found as positive 45 (68%) by using ELISA and as positive 53 (80%) by using EliATM in sera of 66 RA patients. The diagnostic sensitivity and specificity were found 68.2% and 96.9% respectively in the ELISA test and 80.3% and 97.0% in the EliATM test [20].
In another study, 83% sensitivity and 96% specificity were observed for Anti-CCP test, and 70% sensitivity and 86% specificity were observed for RF test in RA patients (<2 years duration). 93% sensitivity and 96% specificity were observed for anti-CCP test, 76.6% sensitivity and 86% specificity were observed for RF test RA patients (3 to 15 years duration) [21].
Bizzaro et al. reported that a consistent number of patients (n = 79, 41.4%) were positive for RF and/or anti-CCP at baseline in RA patients. In the study it is reported that among the RF-positive subjects, 67.1% displayed high RF titers, while in the group of anti-CCP2-positive subjects, an even higher percentage (83.8%) displayed high anti- CCP2 levels in the serum [22].
IgM-RF was found positive in 62.2% of the RA cases and in 16% of controls by Bizzora et al. The sensitivity and specificity of RF respectively were 62% 84% and a good association was found between anti-CCP and IgM-RF in the RA group in their study. They reported that IgM-RF showed a higher sensitivity (62% vs. 41%) and a lower specificity (84% vs. 98%) than anti-CCP and when assayed together anti-CCP and IgM-RF positive, the specificity would increase from 98.7% for anti-CCP alone to 99.6%. They also found a significant correlation between anti-CCP reactivity and early arthritis [23].
RF was tested as positive in 80.0%, and anti-CCP was tested as positive in 82.1% of the RA patients and in by Lin et al. The sensitivity, specificity, PPV, and NPV for anti-CCP antibodies in diagnosing RA were 82.1%, 88.0%, 93.0%, and 71.7% respectively and those for RF were 80.0%, 62.7%, 81.1%, and 61.0% respectively in their study. If anti-CCP or RF positive, sensitivity increased to 88.3%, and if both of them positive, the specificity increased to 94.7%. Anti-CCP was positive in the RF positive RA, RF negative RA, and non-RA patients were 93.1%, 37.9%, and 12.0% respectively [24].
Çiftci et al. reported that sensitivity, specificity, PPV, and NPV in diagnosis of RA for anti-CCP respectively were 0.8657, 1.0000, 1.0000, and 0.9575, and for RF respectively were 0.8955, 0.9113, 0.7692, and 0.9635, and for CRP respectively were 0.7164, 0.6453, 0.4000, and 0.8733, and for ESR respectively were 0.5970, 0.6700, 0.3738, and 0.8344. 44% of the total patients tested positive for CRP, 39.6% for ESR, 28.9% for RF, and 21.5% for anti-CCP in their study. They determined that anti- CCP has the highest correlation percentage with the RA diagnosis among all of the laboratory tests statistically [25].
Anti-CCP has been found diverse ratios positive in RA patients and shown a positive correlation with RF in several studies [15,16,18,23,25]. The diagnostic sensitivity and specificity have shown diversity according even as to the used test methods [20]. Sensitivity has shown diversity 0,41-0,96, and specificity has shown diversity 0,96- 1,00 [20-25]. If anti-CCP or RF positive, sensitivity has increased and if both of them positive, the specificity increased [23,24].
In this study, sensitivity and specificity for anti-CCP are similar to these studies, and if anti-CCP or RF is positive, the sensitivity increased, and if both of them are positive specificity increased. Despite the sensitivity is low, it has high specificity when anti-CCP is high positive especially. There was a positive correlation between anti-CCP and RF positivity, anti-CCP and RF high positivity. As PPV and specificity of anti-CCP are high, NPV and sensitivity are low, so anti-CCP and RF test may be evaluated together.
The highest sensitivity for diagnosis of RA was for ESR, but specificity was not well in this study. The highest specificity for diagnosis of RA was for anti-CCP and RF, if they are high positive especially. Likewise, the highest PPV was for anti-CCP and RF like the specificity. NPV was not well all tests. Anti-CCP antibodies are a highly specific marker for diagnosis of RA patient. The low sensitivity and NPV of the test indicates that a negative anti- CCP antibody test does not exclude disease. Because the test has been high specificity and PPV, it may mean that a positive result of this test increases probability of RA of the patient.
It is concluded that performing anti-CCP test in the diagnosis of RA could be beneficial since it has high specificity and positive predictive value. If this test was performed together with RF, it may be more beneficial.
References
- Wolfe F. The natural history of rheumatoid arthritis. J RheumatolSuppl 1996; 44: 13-22.
- Isomäki H. Long-term outcome of rheumatoid arthritis.Scand JRheumatolSuppl 1992; 95: 3-8.
- Goodyear CS, Tighe H, McInnes IB. Rheumatoid fac- tors and other autoantibodies in rheumatoid arthritis. In: Firestein GS, Budd RC, Harris Jr ED, McInnes IB, Ruddy S,Sergent JS, editors. Kelley’s Textbook of Rheumatology. 8th ed. Philadelphia: W.B. Saunders Company; 2008. [e-book]
- Pincus T, Callahan LF, Sale WG, Brooks AL, Payne LE, Vaughn WK. Severe functional declines, work dis- ability, and increased mortality in seventy-five rheuma- toid arthritis patients studied over nine years. Arthritis Rheum 1984; 27: 864-872.
- Scott DL, Symmons DP, Coulton BL, Popert AJ. Long- term outcome of treating rheumatoid arthritis: results after 20 years. Lancet 1987; 1: 1108-1111.
- Smolen JS, Aletaha D, Koeller M, Weisman M, EmeryP. New therapies for the treatment of rheumatoid arthri- tis. Lancet 2007; 370: 1861-1874.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American RheumatismAssociation 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31: 315-324.
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. An American College of Rheuma- tology/ European League Against Rheumatism collabo-rative initiative 2010 rheumatoid arthritis classification criteria. Arthritis Rheum 2010; 62: 2569-2581.
- Nienhuis RLF, Mandema EA. A new serum factor in patients with rheumatoid arthritis.The perinuclear fac- tor. Ann Rheum Dis 1964; 23: 302-305.
- Young BVJJ, Mallaya RK, Leslie RDG, Clark CJM, Hamblin TJ. Antikeratin antibodies in rheumatoid ar-thritis. Br Med J 1979; 2: 97-99.
- Boekel MAM, Vossenaar ER, van den Hoogen FHJ, van Venrooij WJ. 2002. Autoantibody systems in rheumatoid arthritis: Specificity, sensitivity and diag- nostic value. Arthritis Res 2002; 4: 87-93.
- Van Venrooij WJ, Zendman AJ. Anti-CCP2 antibodies: An overview and perspective of the diagnostic abilities of this serological marker for early rheumatoid arthritis. Clin Rev Allergy Immunol 2008; 34: 36-39.
- Niewold TB, Harrison MJ, Paget SA. Anti-CCP anti- body testing as a diagnostic and prognostic tool in rheumatoid arthritis. Q J Med 2007; 100: 193-201.
- Visser H, le Cessie S, Vos K, Breedveld FC, Hazes JMW. How to diagnose rheumatoid arthritis early: A prediction model for persistent (erosive) arthritis. Ar- thritis Rheum 2002; 46: 357-365.
- Kastbom A, Strandberg G, Lindroos A, Skogh T. Anti- CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRAproject). Ann Rheum Dis 2004; 63: 1085-1089.
- Forslind K, Ahlmen M, Eberhardt K, Hafstrom I, Svensson B. Prediction of radiological outcome in early rheumatoid arthritis in clinical practice: role of antibod- ies to citrullinated peptides (anti-CCP). Ann Rheum Dis 2004; 63: 1090-1095.
- Koivula MK, Aman S, Karjalainen A, Hakala M, Risteli J. Are there autoantibodies reacting against cit-rullinated peptides derived from type I and type II col- lagens in patients with rheumatoid arthritis? Ann Rheum Dis 2005; 64: 1443-1450.
- Kim SK, Bae J, Lee H, Kim JH, Park SH, Choe JY.Greater prevalence of seropositivity for anti-cyclic cit- rullinated peptide antibody in unaffected first-degree relatives in multicase rheumatoid arthritis-affected fam-ilies. Korean J Intern Med 2013; 28: 45-53.
- Deveci K, Deveci H, Bayram KB, Koçyiğit H, Gürgan The Relationship Between Serum Levels of Anti- Cyclic Citrullinated Peptide Antibodies and DiseaseActivity in Patients With Rheumatoid Arthritis. Turk J Phys Med Rehab 2012; 58: 267-271.
- Herold M, Boeser V, Russe E, Klotz W. Anti-CCP: History and its usefulness. ClinDevImmunol 2005; 12: 131-135.
- Manivelavan D, Vijayasamundeeswari CK. Anti-cyclic citrullinated peptide antibody: An early diagnostic and prognostic biomarker of rheumatoid arthritis. J ClinDi- agn Res 2012; 6: 1393-1396.
- Bizzaro N, Bartoloni E, Morozzi G, Manganelli S, Ric- cieri V, Sabatini P, et al. Anti-cyclic citrullinated pep- tide antibody titer predicts time to rheumatoid arthritis onset in patients with undifferentiated arthritis: Results from a 2-year prospective study. Arthritis Res Ther 2013; 15: R16 (pp1-9).
- Bizzaro N, Mazzanti G, Tonutti E, Villalta D, TozzoliR. Diagnostic accuracy of the anti-citrulline antibody assay for rheumatoid arthritis. ClinChem 2001; 47: 1089-1093.
- Lin HK, Lan JL, Chen DY, Chen YH, Huang WN, Hsieh TY, et al. The diagnostic value of anti-cyclic cit- rullinated peptide antibodies and rheumatoid factor in patients with rheumatoid arthritis. Formosan Journal of Rheumatology 2008; 22: 68-73.
- Ciftci IH, Aşik G, Toktaş H, Cufali D, Dundar U, Kar- akece E. An analysis of laboratory characteristics of pa- tients with suspected rheumatoid arthritis. Turk J Rheumatol 2013; 28(1): 27-31.