Research Article - Biomedical Research (2016) Volume 27, Issue 4
Comparison of NRS 2002 and PG-SGA for the assessment of nutritional status in cancer patients
Jiajun Yang1#, Kaitao Yuan2#,Yubao Huang1, Miao Yu2, Xuejun Huang1 , Chao Chen1, Jiyong Fu1,Yingying Shi2, Hanping Shi2*1Department of Colorectal Surgery, Huizhou Municipal Central Hospital, PR China
2Department of General Surgery, The First Affiliated Hospital of Sun Yat-sen University, PR China
#These authors contributed equally
- *Corresponding Author:
- Hanping Shi
Department of Colorectal Surgery
Huizhou Municipal Central Hospital PR China
Accepted on April 07, 2016
Abstract
Purpose: To evaluate the use of the Patient-Generated Subjective Global Assessment (PG-SGA) and the Nutrition Risk Screening 2002 (NRS 2002) for the assessment of nutritional status in patients with common malignant tumors.
Methods: Patients hospitalized in Huizhou Central People’s Hospital from December 2012 to May 2014 were enrolled. The diagnosis with cancer was confirmed by pathological examination and patients received chemotherapy/radiotherapy or surgery. The patients were interviewed by a trained surgeon using NRS 2002 and PG-SGA. Fasting venous blood samples were taken from all enrolled patients, and serum albumin and prealbumin levels were measured. NRS 2002 score ≥ 3 indicated malnutrition risk, and PG-SGA score ≥ 4 indicated malnutrition. The correlations among the scores, serum albumin and prealbumin levels, the body mass index (BMI), the length of hospital stay, and hospitalization cost were analyzed.
Results: 482 patients participated in this study, 242 (50.2%) had NRS 2002 score ≥ 3 and 359 (74.5%) had PG-SGA score ≥ 4. The detection rate of PG-SGA was significantly higher than that of NRS 2002. In patients with serum albumin <35 g/L and prealbumin <0.2 g/L, the detection rates of NRS 2002 and PG-SGA were 67.8% and 93.4%, and 66.4% and 88.8%, respectively, with significant difference. Both NRS 2002 and PG-SGA scores were associated with albumin, pre-albumin, and BMI (P<0.05).
Conclusions: PG-SGA has greater sensitivity than NRS 2002 in assessing nutritional status for patients with common malignancies and is more appropriate for nutritional assessment of cancer patients.
Keywords
NRS 2002, PG-SGA, Cancer, Nutritional assessment.
Introduction
The incidence of malnutrition among hospitalized patients with cancer is high and varies dependent on the tumor location, tumor stage, therapeutic method, and nutritional assessment tool used. Several studies have reported that the occurrence rate of malnutrition related to cancer is 30-87% [1-8]. Malnutrition extends hospital stay, increases health care cost, reduces the quality of life, increases operative risks and complications, and impairs tolerance to chemotherapy and radiotherapy [9]. Some studies have suggested that approximately 20-50% of the mortality factors of cancer patients are related to malnutrition rather than to the cancer itself [10]. The quality of life and prognosis of patients with tumors will be improved and the complications reduced if their nutritional status is improved.
Nutritional therapy for patients with cancer has gradually received clinical attention and become a major component of comprehensive treatment of patients with malignant tumors. However, nutritional therapy only benefits patients who have a nutritional risk or malnutrition. Furthermore, excessive nutritional treatment may expose patients to infection; aggravate their economic burden, and waste medical resources. Therefore, current consensus is that nutritional therapy is beneficial for patients with cancer who have a nutritional risk or malnutrition but is not needed for patients without a nutritional risk or malnutrition. Therefore, timely, accurate, and dynamic assessment of nutritional status is important for nutritional cancer treatment.
There is currently no absolute “gold standard” to determine whether a patient has malnutrition. The diagnosis of nutritional status can be divided into two stages: nutritional screening and nutritional assessment. The aim of the former is to identify malnourished patients or patients with a nutritional risk, especially those who have not yet shown symptoms of malnutrition but who have been found to have a nutritional risk. Nutritional therapy should be combined with clinical treatment for these patients and should be carried out during office visits or admission to a hospital. Nutritional assessment is more extensive for admitted patients; it should comprehensively evaluate nutritional status according to various scale scores, determine whether the patient has malnutrition and its complications, estimate the patient’s nutritional requirements, develop a nutritional therapy plan, and assess the efficacy of nutritional therapy [11]. Nutritional screening has been recommended as a routine for patients admitted to a hospital [12-14]. The European Society for Clinical Nutrition and Metabolism suggested the adoption of the Nutrition Risk Screening 2002 (NRS 2002), the Mini Nutritional Assessment, and the Malnutrition Universal Screening Tool [13]. However, those nutritional screening tools were developed for patients without cancer, and their applicability for patients with cancer remains to be determined. In China, the Chinese Society of Clinical Oncology Specialist Committee of Nutritional Therapy for Cancer recommended that once patients with malignant tumors have clear diagnoses, they should immediately undergo nutritional risk screening. The widely used nutritional assessment tools for patients with malignancy are Patient-Generated Subjective Global Assessment (PG-SGA) and NRS 2002 [12]. This study was performed to compare the use of NRS 2002 and PG-SGA in the assessment of nutritional status of patients with common malignant tumors.
Patients and Methods
Subjects
Patients with common malignant tumors hospitalized in Huizhou Central People’s Hospital from December 2012 to May 2014 were enrolled in this study. The inclusion criteria were as follows: age between 18 and 90 years; a diagnosis of malignancy on the basis of pathological examination; the ability to answer questions without any communication obstacles; the willingness to volunteer for the study; a common malignancy (lung, gastric, liver, colon/rectum, breast, esophageal, cervical, endometrial, nasopharyngeal, pancreatic, ovarian, prostatic, bladder, and brain cancer; malignant lymphoma; and leukemia); and a hospital stay of more than 1 day with no operation before the next morning.
A total of 482 cancer patients were selected, including 255 cases of colon/rectum cancer, 61 of lung cancer, 58 of gastric cancer, 31 of breast cancer, 22 of esophageal cancer, 15 of malignant lymphoma, 10 of cervical cancer, 11 of ovarian cancer, 6 of nasopharyngeal carcinoma, 4 of liver cancer, 3 of bladder cancer, 3 of endometrial cancer, 2 of pancreatic cancer, and 1 of prostatic cancer. 206 were male (42.7%) and 276 were female (57.3%). Their average age was 57.23 ± 12.19 years.
Methods
A single surgeon from a Class III Grade I hospital who had received standard nutritional screening training examined all of the eligible patients. The examination included NRS 2002 and PG-SGA and was completed for each patient within 24 h of hospitalization. The height and body weight were measured to the nearest 0.5 kg and 0.5 cm, respectively, and the patients were in patient dress, did not wear shoes, and fasted that morning. In addition, the changes in body weight during the previous 3 months and the patient’s diet for the previous 2 weeks were recorded. The details of nutritional status assessment standards were as follows:
(1) A body mass index (BMI) <18.5 kg/m2 was considered to be low, a BMI of 18.5-23.9 kg/m2 was considered normal, and a BMI of 24.0-28 kg/m2 was considered overweight [15].
(2) The patients’ nutritional status and nutritional risk were assessed according to NRS 2002, including the severity of illnesses, diet, recent changes in body weight, and somatometry; the total scores ranged from 0 to 7. The patients at least 70 years old received one additional point. The patients were divided into two groups, a group with no nutritional risk (score<3) and a group with a nutritional risk (score ≥ 3). Moreover, patients with a BMI <18.5 kg/m2 were automatically given a score of 3 and assessed as having malnutrition [15].
(3) PG-SGA was divided into two parts and completed independently by the patients and checked by the physician. The patients provided information about historical symptoms, body weight, current mobility, and diet. Total scores were calculated and the results fell into three grades: grade A (total: 0-3) with normal nutrition; grade B (total: 4-8) with moderate malnutrition; and grade C (total: ≥ 9) with severe malnutrition [16].
In addition to completing the questionnaire, in the early morning of the second day after admission, fasting venous blood samples were obtained from each enrolled patient to measure serum albumin and pre-albumin levels.
Statistical analysis
SPSS Statistics 20.0 software package was used for statistical analysis. Rank sum tests, t-tests, and chi-square tests were used to compare the averages and detection rates of the two groups. A matching McNemar chi-square test was used to compare the relevance coincidence of two scales with the same group of subjects (e.g., BMI<18.5). Pearson/Spearman correlation coefficient analysis was conducted for the analysis of the BMI and biochemical indexes (albumin, pre-albumin). The correlation coefficient average was compared by estimating the 95% confidence interval by bootstrap. The inspection level of the hypothesis test was assumed to be ɑ=0.05. In addition, Kolmogorov-Smirnov (n>50) and Shapiro-Wilk (n ≤ 50) tests were used to check normality of quantitative data; the test of normality inspection level was set at ɑ=0.10. P<0.05 was considered as statistically significant.
Results
General data of the patients
Of the 482 patients who participated in the study, 242 (50.2%) scored ≥ 3 on NRS 2002 (147 male and 95 female), and 359 patients (74.5%) scored ≥ 4 on PG-SGA (225 male and 134 female). The patients in the group with malnutrition risk or malnutrition were older than those in the group without malnutrition risk or malnutrition. The gender distribution of the groups when sorted by a PG-SGA score ≥ 4 was statistically significant (P<0.05), whereas that of the groups when classified according to NRS 2002 score ≥ 3 was not (P>0.05).malnutrition (74.5%). When it was used as a “gold standard” to calculate the sensitivity and specificity of NRS 2002 score ≥ 3, the sensitivity was 61.8% and the specificity 83.7%.
Comparison of positive rate between NRS 2002 and PG-SGA
For all 482 patients, the incidence of malnutrition risk was 50.2% according to NRS 2002, while the rate of malnutrition was 74.5% according to PG-SGA. In addition, we found that the positive rate of PG-SGA was significantly higher than that of NRS 2002 in these patients (P<0.05). When a PG-SGA score ≥ 4 was set as the standard for a diagnosis of malnutrition, 359 patients were determined to have malnutrition (74.5%). When it was used as a “gold standard” to calculate the sensitivity and specificity of NRS 2002 score ≥ 3, the sensitivity was 61.8% and the specificity 83.7%.
Correlation of NRS 2002 and PG-SGA scores with serum albumin and prealbumin levels and body mass index
In the patients with serum albumin <35 g/L, the detection rates of NRS 2002 and PG-SGA were 67.8% and 93.4%, respectively, but in the patients with serum prealbumin <0.2 g/L, the detection rates were 66.4% and 88.8%, respectively, the differences were statistically significant. The probability of detecting malnutrition in patients with serum albumin <35 g/L and serum prealbumin <0.2 g/L was higher when PG-SGA scale was used (Tables 1 and 2). The correlation of NRS 2002 and PG-SGA scores with serum albumin and prealbumin levels and BMI was negative (P<0.05). The average difference between the scores and the biochemical index had no statistical significance, indicating that the correlation intensity was the same (Table 3).
| NRS 2002 = 3 | PG-SGA = 4 | P | |
|---|---|---|---|
| Malnutrion 1 | No malnutrion 0 | ||
| Malnutrition risk 1 | 59 (65.6) | 2 (2.2) | <0.001 |
| No malnutrition risk 0 | 25 (27.8) | 4 (4.4) | |
Table 1. Detection rate of NRS 2002 and PG-SGA in cases with serum albumin <35 g/L.
| NRS 2002 = 3 | PG-SGA = 4 | P | |
|---|---|---|---|
| Malnutrition 1 | No malnutrition 0 | ||
| Malnutrition risk 1 | 140 (62.8) | 8 (3.6) | <0.001 |
| No malnutrition risk 0 | 58 (26.0) | 17 (7.6) | |
Table 2. Detection rate of NRS 2002 and PG-SGA in cases with serum prealbumin <0.2 g/L.
| NRS 2002 Score (bootstrap 95% CI) |
PG-SGA Score (bootstrap 95% CI) |
P | |
|---|---|---|---|
| Albumin | -0.297 (-0.335, -0.157)** |
-0.355 (-0.412, -0.252)** |
>0.05 |
| Prealbumin | -0.360 (-0.404, -0.242)** |
-0.396 (-0.424, -0.275)** |
>0.05 |
| BMI | -0.378 (-0.462, -0.304)** |
-0.257 (-0.344, -0.170)** |
>0.05 |
| Spearman correlation coefficient was adopted, and comparison of correlation coefficient average was performed by estimating the 95% confidence interval of the correlation coefficient by bootstrap (midpoint crossing indicated P>0.05; no crossing P<0.05). **Hypothesis testing of each correlation coefficient P<0.01. | |||
Table 3. Correlation of indexes with NRS 2002 and PG-SGA.
Correlation of nutrition scores with hospital stay and hospitalization costs
In chemotherapy or radiotherapy group, the differences in the length of hospital stay and the hospitalization costs between patients with and without malnutrition risk and nutrition scores were significant (P<0.05). Therefore, NRS 2002 and PG-SGA scores could predict patients’ length of hospital stay and hospitalization costs. However, in surgery group, the differences between the malnourished and well-nourished patients evaluated by NRS 2002 and PG-SGA scores were not significant (Tables 4 and 5).
| Operation Group | Chemotherapy or Radiotherapy Group | |||
|---|---|---|---|---|
Positive 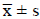 ± s/M
(P25-P75) ± s/M
(P25-P75) |
Neative 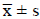 ± s/M
(P25-P75) ± s/M
(P25-P75) |
Positive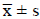 ± s/M
(P25-P75) ± s/M
(P25-P75) |
Negative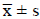 ± s/M
(P25-P75) ± s/M
(P25-P75) |
|
| LOS (days) | 17 (13-21) | 15 (13-18.3) | 6(3.8-12) | 4(3-6) |
| Z | 1.81 | 2.68 | ||
| P | 0.07 | 0.007 | ||
| Costs (×10,000 yuan) | 4.0 (2.8-5.1) | 3.7 (2.3-4.6) | 0.8 (0.5-1.4) | 0.7 (0.5-0.9) |
| Z | 1.60 | 2.09 | ||
| P | 0.11 | 0.036 | ||
Table 4. Comparison of Length of Stay (LOS) and costs of patients evaluated by NRS 2002.
| Operation Group | Chemotherapy or Radiotherapy Group | |||
|---|---|---|---|---|
Positive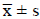 ± s/ ± s/M(P25-P75) |
Negative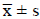 ± s/ ± s/M(P25-P75) |
Positive 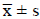 ± s/ ± s/M(P25-P75) |
Negative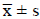 ± s/ ± s/M(P25-P75) |
|
| LOS (days) | 17 (13-21) | 15 (13.5-19.5) | 5 (4-8.5) | 4 (3-6) |
| Z | 0.66 | 4.08 | ||
| P | 0.51 | <0.001 | ||
| Costs (×10,000 yuan) | 4.0 (2.8-5.0) | 3.6 (2.5-4.5) | 0.8 (0.5-1.2) | 0.7 (0.5-0.9) |
| Z | 0.98 | 2.36 | ||
| P | 0.33 | 0.019 | ||
Table 5. Comparison of Length of Stay (LOS) and costs of patients evaluated by PG-SGA.
Discussion
The incidence of malnutrition in patients with malignant tumors has been reported to be 30-87% [1-8]. In this study, we assessed the nutritional status of patients with malignancies, and the incidence of malnutrition was 74.5% evaluated by PGSGA but was only 50.2% evaluated by NRS 2002. The incidence of malnutrition or nutritional risk varies because of different subjects, nutritional screening or assessment tools, tumor locations, stages, and therapeutic aims or methods. There is currently no gold standard for a diagnosis of malnutrition. The ability to estimate a patient’s nutritional status quickly, accurately, conveniently, and non-invasively is the first step in nutritional cancer therapy.
Traditional indices for nutritional status include anthropometric indicators, such as BMI, triceps skin fold thickness, mid-arm muscle circumference, and grip strength, and biochemical indicators, such as serum levels of albumin, pre-albumin, transferrin, and C-reactive protein and total lymphocyte counts. However, each of those nutritional assessment indicators has limitation and could not accurately and comprehensively reflect patients’ nutritional status. Commonly used nutritional screening tools include NRS 2002, Subjective Global Assessment, PG-SGA, Mini-Nutritional Assessment, Malnutrition Universal Screening Tool, Nutrition Risk Index, and Malnutrition Screening Tool. These familiar tools have advantages and disadvantages, but not all of them can be used for patients with malignancies. American Society for Parenteral and Enteral Nutrition and European Society for Parenteral and Enteral Nutrition recommend SGA as a nutritional assessment tool [13,17]. American Nutrition Association recommends PG-SGA as a nutritional screening tool for patients with malignancy. Chinese Society of Clinical Oncology Specialist Committee of Nutritional Therapy for Cancer recommends PG-SGA and NRS 2002 as the nutritional assessment tools for patients with malignancy [11]. No consensus has been achieved about which tool is the most suitable for patients with malignant tumors.
Bauer et al. used SGA and PG-SGA to assess nutritional status. When SGA was set as the standard, the sensitivity of PG-SGA was 98% and the specificity 82%. They concluded that PGSGA was a fast, effective, and reliable tool for the assessment of nutritional status of patients with malignancies [18]. Additional studies have shown that PG-SGA score is closely related to weight loss, the length of hospital stay, quality of life, and energy intake of the patients [19,20]. In this study, PG-SGA and NRS 2002 scores both had weakly positive correlations with the length of hospital stay and hospitalization costs of cancer patients who were hospitalized for chemotherapy or radiotherapy, but were not correlated with those variables in patients hospitalized for surgery. These results may be associated with the fact that comprehensive standardized surgery has not been performed at our hospital, and we have different standards for hospital discharge and surgical methods.
An international multi-center trial validated that NRS 2002 score was closely related to the length of hospital stay, the incidence of complications, and the death rate in patients undergoing gastrointestinal surgery [3]. Schiesser et al. suggested that NRS 2002 score could be applied to predict the incidence of postoperative complications and even postoperative death rate [21]. In this study, the incidence of malnutrition in patients with common malignant tumors was 74.5% and 50.2% when PG-SGA and NRS 2002 were used for nutritional screening, respectively, and the difference was statistically significant. PG-SGA has greater sensitivity in nutritional assessment of patients with common tumors. When a PG-SGA score ≥ 4 was set as a standard for the diagnosis of malnutrition, the sensitivity of an NRS 2002 score ≥ 3 was 61.8% and the specificity was 83.7%.
PG-SGA is mainly based on diet, recent changes in body weight, clinical symptoms, physical examination, and some laboratory measurements. However, in this study PG-SGA score had a weak negative correlation with BMI and serum albumin and prealbumin levels. In contrast, NRS 2002 is based on BMI and albumin level. NRS 2002 has lower sensitivity than PG-SGA because the latter is based on objective laboratory data. In addition, the cost of using the PG-SGA as a nutritional assessment tool was lower, and it is more easily accepted by patients during outpatient reexamination. Nevertheless, PG-SGA failed to screen for malnutrition according to a BMI <18.5 (10.9%), serum prealbumin <35 g/L (6.6%), and serum prealbumin <0.2 g/L (11.2%) in potentially malnourished patients, which indicates that PG-SGA still has some flaws and remains to be improved.
References
- Bo Y, Yao M, Zhang L, Bekalo W, Lu W, Lu Q. Preoperative Nutritional Risk Index to predict postoperative survival time in primary liver cancer patients. Asia Pac J Clin Nutr 2015; 24:591-597.
- Pirlich M, Schutz T, Norman K, Gastell S, Lubke HJ. The Germanhospital malnutrition study. Clin Nutr 2006; 25: 563-572.
- Sorensen J, Kondrup J, Prokopowicz J, Schiesser M, Krähenbühl L. EuroOOPS Study Group.EuroOOPS: An international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr 2008; 27: 340-349.
- Amaral TF, Antunes A, Cabral S, Alves P, and Kent-Smith L. An evaluation of three nutritional screening tools in a Portuguese oncology centre. J Hum Nutr Diet 2008; 21: 575-583.
- Silva FR, de Oliveira MG, Souza AS, Figueroa JN, Santos CS. Factors associated with malnutrition in hospitalized cancer patients: a croos-sectional study. Nutr J, 2015; 14:123.
- Iff S, Leuenberger M, Sterchi A, and Stanga Z. Nutritional management study: screening part. Clin Nutr 2008; 3: 154-154.
- Marin Caro MM, Gomez Candela C, Castillo Rabaneda R, Lourenço Nogueira T, Garcia Huerta M. Nutritional risk evaluation and establishment of nutritional support in oncology patients according to the protocol of the Spanish Nutrition and Cancer Group. Nutr Hosp 2008; 23: 458-468.
- Huhmann MB, Cunningham RS. Importance of nutritional screening in treatment of cancer related weight loss. Lancet Oncol 2005; 5: 334-343.
- Lis CG, Gupta D, Lammersfeld CA, Markman M, Vashi PG. Role of nutritional status in predicting quality of life outcomes in cancer-a systematic review of the epidemiological literature. Nutr J 2012; 2012: 11–27.
- Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996; 12: 15-19.
- CSCO Tumor Nutritional Therapy Specialist Committee. Consensus of nutritional therapy expert in malignant tumor patients J Clin Oncol 2012; 1: 59-73.
- ASPEN Board Directors and the Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. Enteral Nutr 2002; 26: 1-138.
- Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003; 22: 415-421.
- Elia M, Zellipour L, Stratton RJ. To screen or not to screen for adult malnutrition? Clin Nutr 2005; 24: 867-884.
- Shi H-P, Ling W-H, Li W. Nutritional oncology. Beijing: People’s Medical Publishing House 2012; 529-537.
- Jiang Z, Lining J. Nutritional screening and assessment of malignant tumors. J Clin Surg 2006; 4: 201-203.
- Weimann A, Braga M, Harsanyi L, Laviano A, Ljungqvist O, Soeters P. ESPEN guidelines on enteral nutrition: surgery including organ transplantation. Clin Nutr 2006; 25: 224-244.
- Bauer J, Capra S, Ferguson M. Use of the scored patient generated subjective global assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr 2002; 6: 779-785.
- Isenring E, Bauer J, Capra S. The scored patient-generated subjective global assessment (PG-SGA) and its association with quality of life in ambulatory patients receiving radiotherapy. Eur J Clin Nutr 2003; 57: 305-309.
- Gabrielson DK, Scaffidi D, Leung E, Stoyanoff L, Robinson J, Nisenbaum R, Brezden-Masley C, Darling PB. Use of an abridged scored Patient-Generated Subjective Global Assessment (abPG-SGA) as a nutritional screening tool for cancer patients in an outpatient setting. Nutr Cancer 2013; 65:234-239.
- Schiesser M, Müller S, Kirchhoff P, Breitenstein S, Schäfer M, Clavien PA. Assessment of a novel screening score for nutritional risk in predicting complications in gastrointestinal surgery. Clin Nutr 2008; 27: 565-570.