Research Article - Biomedical Research (2017) Volume 28, Issue 5
Comparison of human papillomavirus genotype distributions in cervical intraepithelial neoplasia and cervical cancer
Xiaolin Li, Xiaojie Wan, Feiyun Zheng, Haiyan Zhu, Xuejie Zhu, Jianqin Yu*Department of Gynecology and Obstetrics, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, PR China
- *Corresponding Author:
- Jianqin Yu
Department of Gynecology and Obstetrics
The First Affiliated Hospital of Wenzhou Medical University PR China
Accepted on October 27, 2016
Abstract
We evaluated the genotype distributions of human papillomavirus (HPV) in cervical intraepithelial neoplasia (CIN) and cervical cancer (CC) cases. Cervical exfoliated cells from 490 patients (125, 231, and 134 patients in groups CIN1, CIN2+, and CC, respectively) were tested to determine the HPV genotypes. The positive rates were statistically analyzed to determine the correlations between HPV and disease severity. Average age of subjects in the CIN1, CIN2+, and CC groups was 44.3 ± 10.3, 44.1 ± 11.8, and 52.3 ± 10.9 years, respectively. The average age in group CC was higher than that in groups CIN2 + and CIN1 (P<0.001). The following HPV subtypes were the most common in groups CIN1, CIN2+, and CC, respectively: HPV16, HPV58, HPV18, and HPV33; HPV16, HPV58, HPV52, and HPV33; and HPV16, HPV18, HPV58, and HPV33. Group CIN2+ showed a higher positive rate of HPV16/52 and lower positive rate of HPV51 than group CIN1 (P<0.05). Group CC showed a higher positive rate of HPV16 and lower positive rate of HPV33/52/58 than group CIN2+ (P<0.05). Multiple infection rates of HPV were 28.8%, 36.8%, 16.4% in groups CIN1, CIN2+, and CC, respectively, and that in group CC was lower than in groups CIN2+ and CIN1 (P<0.001). HPV16/18 are the most important predisposition factors for CC and HPV33/52/58 showed weak carcinogenicity but belonged to high-risk subtypes of CC; therefore, attention should be given to HPV16/18 and HPV33/52/58 infection.
Keywords
Cervical intraepithelial neoplasia, Cervical cancer, Human papillomavirus, Genotyping.
Introduction
Cervical cancer (CC) is the third most prevalent malignant tumor in women [1] and the most common gynecologic malignancy. Approximately 500,000 new cases and 250,000 deaths from CC occur each year [1]. Continuous infection with high-risk HPV (HR-HPV) is the main cause of cervical precancerous lesions and invasive cervical cancer [2]. At least 15 HPV genotypes (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82) have been identified as HR-HPV [3], among which HPV16 exhibits the closest relationship with CC, followed by HPV18 [4]. Currently, HPV vaccines are applied worldwide mainly for HPV16/18 and HPV 6/11/16/18 [5]. However, in different geographic regions and populations, the distributions of HPV subtypes are different [6]. In northeast China, the most common HPV genotypes are HPV-16 (28%), HPV-58 (14%), and HPV-52 (14%), and HPV-18 accounts for only 8% [7,8]. In western China, the most common HPV genotypes are HPV-16, HPV-33, and HPV-58 [9]. In Southeastern China, the most common genotypes are HPV-52, HPV-16, and HPV-58 [10]. Vaccines against HPV16/18 have limited effects on other HR-HPV infections [11]. Thus, an effective vaccine should contain the HPV subtypes with the highest local prevalence. Here, we detected the infection rates of HPV16/18 and other HR-HPV subtypes in cervical precancerous lesions and CC to explore the most common and highly carcinogenic HPV types in CC. Our results provide a basis for CC vaccine development and pre-judgment of cervical diseases by gynecologists in China.
Materials and Methods
Materials
Cervical exfoliated cells sampled from 490 CIN or CC patients, histologically diagnosed by cervical biopsy or cervical conization in the First Affiliated Hospital of Wenzhou Medical University from June 2015 to February 2016, were selected as study subjects, including 125 cases of CIN1, 231 cases of CIN2-3 (group CIN2+), and 134 cases of CC. No patients had a history of HPV vaccination. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Wenzhou Medical University. Written informed consent was obtained from all participants.
Pathological diagnosis
The tissue sections were subjected the hematoxylin-eosin staining and then diagnosed by two experienced pathologists in our hospital based on WHO classification. The diagnosis was further confirmed after review. When results of cervical biopsy and cervical conization were inconsistent, results showing a higher histological grade were considered for the final diagnosis.
HPV genotyping
After histological diagnosis, cell preservation solution (TEGEN, Shanghai, China) was used to collect the cervical exfoliated cells, followed by in vitro PCR amplification and subsequent DNA reverse dot blot-combined DNA chip technology to determine HPV subtypes of the cervical cell specimens for genotype identification. The specific detection operations included extraction of DNA, PCR amplification, hybridization, washing of the film, and coloration.
Statistical analysis
SPSS 17.0 software was used for statistical analysis (SPSS, Inc., Chicago, IL, USA), percentages (count data) were compared using the χ2 test. Intergroup comparison of measurement data was performed by the t-test. P-values<0.05 were considered statistically significant.
Results
Age group of patients
Mean age of patients was 44.3 ± 10.3 (24 to 73) years in Group CIN1, 44.1 ± 11.8 (21 to 79) years in Group CIN2+, and 52.3 ± 10.9 (32 to 85) years in Group CC. The patients in group CC were older than those in groups CIN2+ and CIN1 (t=6.561, P=0.000; t=6.047, P=0.000); subjects in groups CIN2+ and CIN1 showed no significant difference in age (t=0 163, P=0.871).
HPV distribution and positive rates
Of the 125 patients in group CIN1, 109 were HPV-positive (87.2%), including 145 HPV types (one sample may have contained more than one HPV type). Of the 231 patients in group CIN2+, 212 were HPV-positive (91.8%), including 318 HPV types. The four most common HPV types and their positive rates in these two groups were: group CIN1: HPV16 (24.8%), HPV58 (17.6%), HPV18 (8.8%), HPV33, and HPV51 (7.2%); group CIN2+: HPV16 (44.2%), HPV58 (24.7%), HPV52 (16.5%), and HPV33 (13.0%). Group CIN1 included 17 cases of low-risk HPV (4 cases of simple HPV 11 infection, 1 case of simple HPV81 infection) and group CIN2+ included 13 cases of low-risk HPV (1 case of simple HPV6 infection, and one case of simple HPV11 infection); the remaining cases were infected with other HR-HPV types simultaneously. A comparison of the total positive rates of low-risk HPV between the two groups showed a significant difference (χ2=6.680, P=0.01); however, a comparison of the positive rate of simple low-risk HPV showed no significant difference (P=0.055, Table 1).
| Typing | CIN1 (n=125) | CIN2+(n=231) | CIN2+VS CIN1 | |||
|---|---|---|---|---|---|---|
| n | Positive rate (%) | n | Positive rate (%) | χ2 | P | |
| HR | ||||||
| 16 | 31 | 24.8 | 102 | 44.2 | 12.985a | 0.000** |
| 18 | 11 | 8.8 | 24 | 10.4 | 0.231a | 0.631 |
| 31 | 6 | 4.8 | 7 | 5.3 | 0.039 a | 0.843 |
| 33 | 9 | 7.2 | 30 | 13 | 2.785 a | 0.095 |
| 35 | 3 | 2.4 | 6 | 2.6 | 0.000b | 1 |
| 39 | 6 | 4.8 | 5 | 2.2 | 1.104 b | 0.293 |
| 45 | 0 | 0 | 5 | 2.2 | ||
| 51 | 9 | 7.2 | 4 | 1.7 | 5.427 b | 0.020* |
| 52 | 7 | 5.6 | 38 | 16.5 | 8.647 a | 0.003* |
| 53 | 8 | 6.4 | 9 | 3.9 | 1.118 a | 0.29 |
| 56 | 3 | 2.4 | 5 | 2.2 | 0.000 b | 1 |
| 58 | 22 | 17.6 | 57 | 24.7 | 2.352 a | 0.125 |
| 59 | 3 | 2.4 | 3 | 1.3 | - | 0.427c |
| 66 | 3 | 2.4 | 4 | 1.7 | - | 0.700c |
| 68 | 7 | 5.6 | 6 | 2.6 | 1.313 b | 0.252 |
| LR | ||||||
| 6 | 0 | 0 | 4 | 1.7 | ||
| 11 | 6 | 4.8 | 1 | 0.4 | ||
| 42 | 1 | 0.8 | 0 | 0 | ||
| 43 | 1 | 0.8 | 1 | 0.4 | ||
| 44 | 3 | 2.4 | 1 | 0.4 | ||
| 55 | 1 | 0.8 | 1 | 0.4 | ||
| 61 | 2 | 1.6 | 2 | 0.9 | ||
| 81 | 3 | 2.4 | 2 | 0.9 | ||
| 83 | 0 | 0 | 1 | 0.4 | ||
Note: 1. The positive rate of different types in the patients with multiple infections were repeatedly calculated. 2. **P<0.001,*P<0.05. 3. a: Pearson χ2, b: successively corrected χ2, c: P obtained by Fisher exact statistics; when the positive rate of one group was 0, no statistical process was performed.
Table 1. HPV subtype distributions and positive rates in CIN.
Of the 134 patients in group CC, 119 were HPV-positive (88.8%), including 148 HPV types. The four most common HPV subtypes and their positive rates in CC were HPV16 (59.7%), HPV18 (15.7%), HPV58 (6.0%), and HPV33 (5.2%). The HPV-positive rate of adenocarcinoma was 64.0%, which was lower than that of squamous cell carcinoma (94.5%) (P<0.01). The four most common HPV types in squamous cell carcinoma were HPV16 (68.8%), HPV18 (10.1%), HPV58 (7.3%), and HPV33 (6.4%). The four most common HPV subtypes in adenocarcinoma were HPV18 (40.0%), HPV16 (20.0%), HPV58 (4.0%), and HPV43 (4.0%, Table 2).
| Typing | CC (n=134) | Cervical squamous carcinoma (n=109) | Cervical adenocarcinoma (n=25) | CCvsCIN2+ | ||||
|---|---|---|---|---|---|---|---|---|
| n | Positive rate (%) | n | Positive rate (%) | n | Positive rate (%) | χ2 | P | |
| HR | ||||||||
| 16 | 80 | 59.7 | 75 | 68.8 | 5 | 20 | 8.198 a | 0.004* |
| 18 | 21 | 15.7 | 11 | 10.1 | 10 | 40 | 2.189 a | 0.139 |
| 26 | 1 | 0.7 | 1 | 0.9 | 0 | 0 | - | - |
| 31 | 3 | 2.2 | 3 | 2.8 | 0 | 0 | 0.013 b | 0.909 |
| 33 | 7 | 5.2 | 7 | 6.4 | 0 | 0 | 5.611 a | 0.018* |
| 35 | 1 | 0.7 | 1 | 0.9 | 0 | 0 | - | 0.430 c |
| 39 | 2 | 1.5 | 2 | 1.8 | 0 | 0 | - | 1.000c |
| 45 | 5 | 3.7 | 5 | 4.6 | 0 | 0 | 0.304 b | 0.581 |
| 52 | 5 | 3.7 | 5 | 4.6 | 0 | 0 | 13.200 a | 0.000** |
| 56 | 2 | 1.5 | 2 | 1.8 | 0 | 0 | - | 1.000 c |
| 58 | 9 | 6.7 | 8 | 7.3 | 1 | 4 | 18.465 a | 0.000** |
| 59 | 1 | 0.7 | 1 | 0.9 | 0 | 0 | - | 1.000 c |
| 66 | 1 | 0.7 | 3 | 2.8 | 0 | 0 | - | 0.656 c |
| 68 | 1 | 0.7 | 1 | 0.9 | 0 | 0 | - | 0.430 c |
| 82 | 2 | 1.5 | 2 | 1.8 | 0 | 0 | ||
| LR | ||||||||
| 82 | 2 | 1.5 | 2 | 1.8 | 0 | 0 | ||
| 43 | 1 | 0.7 | 0 | 0 | 1 | 4 | ||
| 55 | 2 | 1.5 | 2 | 1.8 | 0 | 0 | ||
| 61 | 3 | 2.2 | 3 | 2.8 | 0 | 0 | ||
Note: 1. The positive rate of different types in the patients with multiple infections were repeatedly calculated. 2. **P<0.001,*P<0.05. 3. a: Pearson χ2, b: successively corrected χ2, c: P obtained by Fisher exact statistics; when the positive rate of one group was 0, no statistical process was performed.
Table 2. HPV subtype distributions and positive rates in CC.
.Comparison of HPV positive rates
A comparison of groups CIN2 + and CIN1 showed that the former had higher positive rates of HPV16 and HPV52 (P<0.05), but lower positive rate of HPV51 (P<0.05); the positive rates of HPV18, HPV33, and HPV58 showed no significant difference (P>0.05). A comparison of groups CC and CIN2+ showed that the former had a higher positive rate of HPV16 (P<0.05) but lower positive rates of HPV33, HPV 52, and HPV58 (P<0.05).
Comparison of simple- and multiple-infection and negative rates
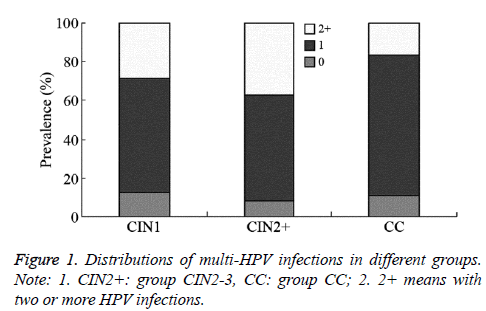
Simple-infection rates in groups CIN1, CIN2+, and CC were 58.4% (73/125), 55.0% (127/231), and 72.4% (97/134), respectively (χ2=0.386, P=0.535 (between CIN1 and CIN2+), χ2=10.842, P=0.001 (between CIN2+ and CC)). The multipleinfection rates in groups CIN1, CIN2+, and CC were 28.8% (36/125), 36.8% (85/231), and 16.4% (22/134) (χ2=2.312, P=0.128 (between CIN1 and CIN2+), χ2=16.996, P=0.000 (between CIN2+ and CC)); the HPV-negative rates were 12.8% (16/125), 8.2% (19/231), and 11.2% (15/134), and comparison of these three groups showed P>0.05 (Figure 1).
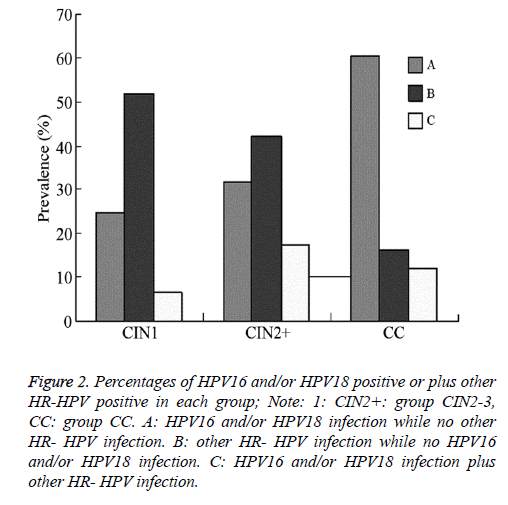
The proportion of patients with HPV16 and/or HPV18 infection with no other HR-HPV infection in groups CIN1, CIN2+, and CC were 24.8% (31/125), 31.6% (73/231), and 60.4% (81/134), respectively. The proportion in group CIN2 + was lower than that in group CC (χ2=28.932, P=0.000); however, the difference between groups CIN2 + and CIN1 was not significant (χ2=1.815, P=0.178). The proportion of patients with other HR- HPV infections but no HPV16 and/or HPV18 infection in groups CIN1, CIN2+, and CC was 52.0% (65/125), 42.0% (97/231), and 16.4% (22/134), respectively. The proportion in group CIN2 + was higher than that in group CC (χ2=25.241, P=0.000); however, the difference between groups CIN2+ and CIN1 was not significant (χ2=3.276, P=0.070). The proportion of patients with HPV16 and/or HPV18 infection plus other HR-HPV infection in groups CIN1, CIN2+, and CC was 6.4% (8/125), 17.3% (40/231), and 11.9% (16/134), respectively. The proportion in group CIN2+ was higher than that in group CIN1 (χ2=8.285, P=0.004); however, the difference between groups CIN2+ and CC was not statistically significant (χ2=2.361, P=0.124, Figure 2).
Figure 2: Percentages of HPV16 and/or HPV18 positive or plus other HR-HPV positive in each group; Note: 1: CIN2+: group CIN2-3, CC: group CC. A: HPV16 and/or HPV18 infection while no other HR- HPV infection. B: other HR- HPV infection while no HPV16 and/or HPV18 infection. C: HPV16 and/or HPV18 infection plus other HR- HPV infection.
Discussion
The occurrence and development of CC involves quantitative and qualitative changes, from gradual changes to mutations, and progresses from of CIN- carcinoma in situ to early invasive cancer and then invasive cancer. CIN often occurs in women aged 25-35 years, and the highest CC incidence occurs at 40-54 years [12]. In this study, the average age of CIN patients was approximately 44 years, whereas that of CC patients was about 52 years. The average age of CC patients was higher than that of other patients; therefore, CC screening in this region should be increased for early detection and timely treatment. Persistent infection of HR-HPV is necessary for the occurrence and development of CIN and CC [13]. HPV genotyping detection is the only method to identify persistent HPV infection in females. Because there are various HPV types that show differing carcinogenic potencies, improving HR-HPV genotyping detection in women in the high-risk CC age range may be an effective means for CC screening and CIN follow-up in obstetrics and gynecology.
Different regions show different HPV subtypes of CC, and the HPV16 subtype is the most common with an infection rate of 40-60%; HPV18 accounts for 10-20% of cases [14]. A metaanalysis analyzing 984 Japanese CC patients found that the three most common HPV subtypes were HPV16 (44.8%), HPV18 (14.0%), and HPV52 (7.0%) [15]. In Chinese CC patients, the HPV infection is mainly caused by HPV16 and 58; of these, HPV16 shows the closest relationship with cervical squamous cell carcinoma and HPV18 is the most likely to cause cervical adenocarcinoma [16]. In this study, HPV16 was the most prevalent subtype in CC, followed by HPV18, HPV58, and HPV33. HPV16 was the most prevalent subtype in cervical squamous cell carcinoma, followed by HPV18, HPV58, and HPV33. HPV18 was the most prevalent subtype in CC, followed by HPV16 and HPV58. These results indicate that in addition to HPV16 and HPV18 in CC, HPV58 and HPV33 were the most common subtypes. Therefore, when detecting HPV16 and HPV18 infection, HPV58 and HPV33 infection should also be evaluated. Currently, most HPV screening programs only detect the HPV6, 11, 16, and 18 subtypes, and current HPV vaccines only target these four subtypes [5]. If vaccines have limited cross-protection toward HPV58 and HPV33, the chances of HPV58 and HPV33- positive CC may be increased.
In this study, the HPV-positive rate of cervical adenocarcinoma (64.0%) was lower than that of squamous cell carcinoma (94.5%) and those reported in other similar studies (65.6-82.0%) [17,18]. The low detection rate of HPV DNA in adenocarcinoma may be because the HPV L1 and E6 genes were cleaved when HPV integrated into the host genome, improper cervical cell sampling, or the degradation of cell samples contained low levels of HPV DNA [15]. In cervical adenocarcinoma, low HPV-positive can also be attributed to the presence of HPV-independent glandular lesions. Recently, gastric adenocarcinoma has been proposed as another subtype of cervical adenocarcinoma, and was found to be independent of HPV infection [19]. Nonetheless, the number of cervical adenocarcinoma cases was low in this study, and larger samples sizes need to be tested to confirm this hypothesis. The results of this study showed that the HPV types of CIN were similar to those determined by Onuki et al. [20], where the four most common types in the CIN1 group were HPV16, HPV58, HPV18, and HPV33 and the four most common types in the CIN2+ group were HPV16, HPV58, HPV52, and HPV33. These results suggest that HPV16 played a major role in CIN and that infection with HPV58, HPV52, and HPV33 were also common.
A recent prospective study proposed seven HPV subtypes (HPV16, 18, 31, 33, 35, 52, and 58) as high risk factors for the progression of cervical lesions [21]. This study found that compared with CIN2+, CC showed a higher positive rate of HPV16, supporting that continuous HPV16 infection increases carcinogenicity. In contrast, we found that CC had lower positive rates of HPV33, HPV52, and HPV58 than that of CIN2+, indicating that when CIN progresses to CC, HPV33, HPV52, and HPV58 show low carcinogenicity. We observed an increased simple HPV infection rate from CIN to CC lesions; however, multiple infection rates decreased, which does not support the view that multiple infections are associated with the severity of cervical lesions. In contrast, a single infection appears to be associated with the severity of cervical lesions, as described by Hou et al. [22]. This shows that multiple infections have lower carcinogenicity than single infection.
This study showed that group CC included a larger number of simple HPV16- and/or HPV18-infected patients than group CIN1 and CIN2+; however, the number of patients with other HR-HPV infections was lower, indicating that HPV16/18 have higher carcinogenicity than other HR-HPV subtypes during the development from CIN to CC. Some studies have proposed that cervical diseases associated with HPV16 infection subside with more difficultly than when combined with other HR-HPV infections [23]. In summary, persistent infection with HPV16/18 is the most important predisposition factor in CC; therefore, these subtypes have important clinical significance. HPV33, HPV52, and HPV58 are also high-risk subtypes in cervical precancerous lesions and CC; however, compared with HPV16/18, HPV33, HPV52, and HPV58 show lower carcinogenicity. The sample size in this study was small. Large-sample multi-center studies are required in the future that should focus on polymorphisms in regional HPV33, HPV52, and HPV58. Moreover, whether HPV16/18 vaccines show better protection against simultaneous infection with HPV33, HPV52, and HPV58 should be determined.
References
- Bayu H, Berhe Y, Mulat A, Alemu A. Cervical Cancer Screening Service Uptake and Associated Factors among Age Eligible Women in Mekelle Zone, Northern Ethiopia, 2015: A Community Based Study Using Health Belief Model. PLoS One 2016; 11: e0149908.
- Tamalet C, Halfon P, Retraite LL, Grob A, Leandri FX, Heid P, Sancho-Garnier H, Piana L. Genotyping and follow-up of HR-HPV types detected by self-sampling in women from low socioeconomic groups not participating in regular cervical cancer screening in France. J Clin Virol 2016; 78: 102-107.
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348: 518-527.
- Thomsen LT, Frederiksen K, Munk C, Junge J, Iftner T, Kjaer SK. Long-term risk of cervical intraepithelial neoplasia grade 3 or worse according to high-risk human papillomavirus genotype and semi-quantitative viral load among 33,288 women with normal cervical cytology. Int J Cancer 2015; 137: 193-203.
- Skinner SR, Apter D, De Carvalho N, Harper DM, Konno R, Paavonen J, Romanowski B, Roteli-Martins C, Burlet N, Mihalyi A, Struyf F. Human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for the prevention of cervical cancer and HPV-related diseases. Expert Rev Vaccines 2016; 15: 367-387.
- Wei H, Wang N, Zhang Y, Zhang J, Wang S, Zhang S. Distribution of various types of low-risk human papillomavirus according to cervical cytology and histology in northern Chinese women. Int J Gynaecol Obstet 2014; 126: 28-32.
- Sun ZR, Ji YH, Zhou WQ, Zhang SL, Jiang WG, Ruan Q. Characteristics of HPV prevalence among women in Liaoning province, China. Int J Gynaecol Obstet 2010; 109: 105-109.
- Li Y, Wang Y, Jia C, Ma Y, Lan Y, Wang S. Detection of human papillomavirus genotypes with liquid bead microarray in cervical lesions of northern Chinese patients. Cancer Genet Cytogenet 2008; 182: 12-17.
- Dai Y, Huang YS, Tang M, Lv XP, Li TY, Yin YB. Distribution and clinical significance of human papillomavirus subtypes in Shenzhen city, People's Republic of China. Int J Gynecol Cancer 2008; 18: 295-299.
- Ye J, Cheng X, Chen X, Ye F, Lu W, Xie X. Prevalence and risk profile of cervical Human papillomavirus infection in Zhejiang Province, southeast China: a population-based study. Virol J 2010; 7: 66.
- Wheeler CM, Castellsagué X, Garland SM, Szarewski A, Paavonen J, Naud P, Salmerón J, Chow SN, Apter D, Kitchener H, Teixeira JC, Skinner SR, Jaisamrarn U, Limson G, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, Bosch FX, Harper DM, Huh W, Hardt K, Zahaf T, Descamps D, Struyf F, Dubin G, Lehtinen M. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13: 100-110.
- Marquardt K, Stubbe M, Broschewitz U. Cervical cancer in Mecklenburg-Western Pomerania: Tumor stage, histological tumor type, age and screening participation of 985 patients. Pathologe 2016; 37: 78-83.
- Bottari F, Sideri M, Gulmini C, Igidbashian S, Tricca A, Casadio C, Carinelli S, Boveri S, Ejegod D, Bonde J, Sandri MT. Comparison of Onclarity Human Papillomavirus (HPV) Assay with Hybrid Capture II HPV DNA Assay for Detection of Cervical Intraepithelial Neoplasia Grade 2 and 3 Lesions. J Clin Microbiol 2015; 53: 2109-2114.
- Harris SL, Thorne LB, Seaman WT. Association of p16 (INK4a) overexpression with improved outcomes in young patients with squamous cell cancers of the oral tongue. Head Neck 2011; 33: 1622-1627.
- Miura S, Matsumoto K, Oki A, Satoh T, Tsunoda H, Yasugi T, Taketani Y, Yoshikawa H. Do we need a different strategy for HPV screening and vaccination in East Asia? Int J Cancer 2006; 119: 2713-2715.
- Herbert J, Coffin J. Reducing patient risk for human papillomavirus infection and cervical cancer. J Am Osteopath Assoc 2008; 108: 65-70.
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11: 1048-1056.
- Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer 2011; 128: 927-935.
- Mikami Y, Mccluggage WG. Endocervical glandular lesions exhibiting gastric differentiation: an emerging spectrum of benign, premalignant, and malignant lesions. Adv Anat Pathol 2013; 20: 227-237.
- Onuki M, Matsumoto K, Satoh T, Oki A, Okada S, Minaguchi T, Ochi H, Nakao S, Someya K, Yamada N, Hamada H, Yoshikawa H. Human papillomavirus infections among Japanese women: age-related prevalence and type-specific risk for cervical cancer. Cancer Sci 2009; 100: 1312-1316.
- Matsumoto K, Oki A, Furuta R, Maeda H, Yasugi T, Takatsuka N, Mitsuhashi A, Fujii T, Hirai Y, Iwasaka T, Yaegashi N, Watanabe Y, Nagai Y, Kitagawa T, Yoshikawa H. Predicting the progression of cervical precursor lesions by human papillomavirus genotyping: a prospective cohort study. Int J Cancer 2011; 128: 2898-2910.
- Hou R, Xu C, Zhang S, Wu M, Zhang W. Distribution of human papillomavirus genotype and cervical neoplasia among women with abnormal cytology in Beijing, China. Int J Gynaecol Obstet 2012; 119: 257-261.
- Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol 2009; 113: 18-25.