- Biomedical Research (2014) Volume 25, Issue 3
Comparison of blood sugar and glycosylated hemoglobin in type 2 diabetic patients of Chinese provinces at different altitudes.
Haibing Ju1*, Liping Yang2, Jian Fan3, Zizheng Shu11Department of Endocrinology, Kunming General Hospital of Chengdu Military Area Command, Kunming 650032, China
2Department of Medicine, People's Hospital of Lijiang City, Lijiang 674100, China
3Department of Medicine, People's Hospital of Jinghong City, Jinghong 666100, China
- *Corresponding Author:
- Haibing Ju
Department of Endocrinology
Kunming General Hospital of Chengdu
Military Area Command
Kunming 650032, China
Accepted May 06 2014
Abstract
This work aims to compare the levels of blood sugar, hemoglobin and glycosylated hemoglobin between type 2 diabetic patients of different gender and age, living at different altitudes in Yunnan, China. We selected 410 patients with type 2 diabetes from Kunming, Lijiang and Jinghong of Yunnan Province and compared their levels of fasting blood sugar, 2- hour postprandial blood sugar, hemoglobin and glycosylated hemoglobin. We categorized our findings in terms of gender, age, and the altitude where the patients normally inhabit. We analyzed the Pearson correlation coefficient of single factor of altitude and glycosylated hemoglobin. The fasting blood sugar, 2-hour postprandial blood sugar and glycosylated hemoglobin were not statistically different between the patients with type 2 diabetes of different genders and ages living at different altitudes, but the hemoglobin concentration were found to be statistically different. No significant relationship was, however, observed between the levels of altitude and glycosylated hemoglobin. Levels of altitude, gender and age showed effects on hemoglobin concentration in diabetic patients, but they failed to exert any significant effects on blood sugar and glycosylated hemoglobin concentrations.
Keywords
Type 2 diabetes mellitus, Glycosylated hemoglobin, Altitude.
Introduction
In the past several decades, detection of glycosylated hemoglobin (HbA1C) has become a standard in diabetic patient care. With the increasing understanding of the role of HbA1C in diabetes management, HbA1C has become one of the hotspots in clinical research today. HbA1C reflects the average blood sugar level of patients over a period of time, and large-scale studies have shown that HbA1C is a test index with the highest correlation with diabetic retinopathy, diabetic nephropathy and diabetic neuropathy [1]. In addition, HbA1C is more stable and affected by fewer factors than fasting blood sugar and 2-hour postprandial blood sugar [2] and is becoming increasingly used in clinical applications. HbA1C is not only used as a monitoring index to control blood sugar in patients with diabetes, but is also used for screening and diagnosis of diabetes [3]. The combination of HbA1C, blood glucose point values and dynamic blood sugar monitoring provides health care providers with a better understanding of the patient’s ability to control his or her blood sugar level, as well as a means to monitor and predict of the risks associated with chronic diabetes [4]. In addition to longterm blood sugar levels, whether other non-pathological factors such as altitude, ethnicity, and gender affect the measured levels of HbA1C is still an open question [5].
At present, China has not yet adopted HbA1C as an index for screening and diagnosis of diabetes, mainly because there is not a standardized method for detecting HbA1C across various laboratories. Furthermore, the vast geographical variability of each province in China may also result in discrepancies in measured HbA1C values. Therefore, further clarification of the effect of non-pathological factors on the measured levels of HbA1C will help to promote the clinical application of HbA1C in China. Early identification of the desired threshold for diagnosis or screening diabetes by using HbA1C in China has important clinical significance [6,7].
Yunnan is a multi-ethnic province, including Han Chinese and 25 minority ethnic groups. At the same time, Yunnan Province has complex terrain with an average elevation of about 2000m above sea level. However, the elevation differs greatly throughout the province with the lowest point being 76m above the sea level, and the highest elevation being 6740m above the sea level. In this study, patients with type 2 diabetes were selected from three different elevations in order to assess the difference in blood sugar and glycosylated hemoglobin between the patients with diabetes living at different altitudes of Chinese provinces.
Subjects and Methods
Subjects
Between January 2011 and June 2012, unadjusted patients with type 2 diabetes who lived in Kunming, Lijiang and Jinghong, at different altitudes of Yunnan Province, for more than 5 years were selected. The patients had been diagnosed with diabetes for more than six months and received glucose lowering treatment for more than 3 months. Elimination criteria: Blood disease such as hemophthisis and hemoglobinopathy; acute complications of diabetes such as diabetic ketoacidosis, hyperosmolar coma and hypoglycemia; patients under a state of physical stress such as acute myocardial infarction, acute cerebral infarction, infection, trauma, and operation. Based on these criteria, 101 patients were selected from an elevation of 500m above the sea level in Jinghong city, 208 patients were selected from an elevation of 1900m above the sea level in Kunming city, and 101 patients were selected from an elevation of 2420m above the sea level in Lijiang city. This study was conducted in accordance with the declaration of Helsinki and approval from the Ethics Committee of Kunming General Hospital of Chengdu Military Area. Written informed consent was obtained from all participants.
Methods
Eligible patients (410 totals) with type 2 diabetes mellitus were phlebotomized at three different times to test their fasting and 2-hour postprandial blood sugar. Their serum glucose was tested with the oxidase method and the aver age value of three tests was taken as the fasting blood glucose and 2-hour postprandial blood glucose. The fasting patients were phlebotomized in the early morning to test their glycosylated hemoglobin with the immunoturbidimetric method using a fully automatic blood cell analyzer. The level of hemoglobin was considered normal if the values ranged between 110g/L-160g/L, and considered high if the value showed >160g/L.
Statistical analysis
SPSS13 software was used for statistical analysis and the measured data were expressed as the mean + standard deviation. The test was used for comparison between two groups, Pearson correlation analysis was used for correlation analysis, Kruskal-Wallis test was used to compare multiple independent samples at different altitudes and ethnicity, Nemenyi test was used for pairwise comparison, and we considered any differences to be statistically significant when P<0.05.
Results
Clinical data
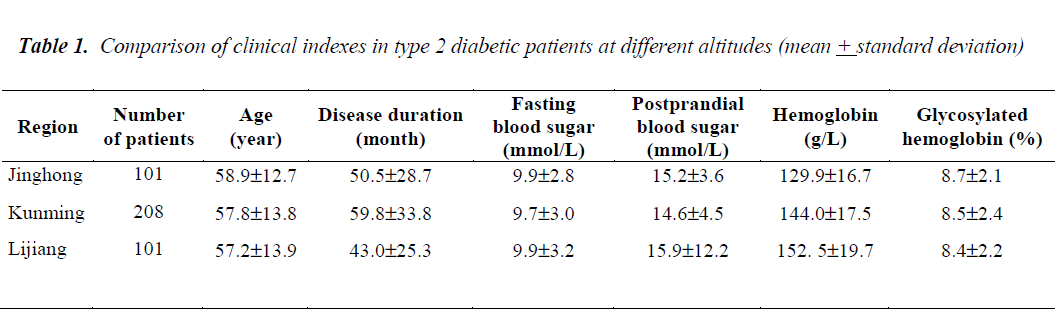
Table 1 shows that using the Kruskal-Wallis test of multiple independent samples, patient factors such as age (kw=0.48 P=0.79), disease duration (kw=3.38 P =0.19), fasting blood sugar (kw=1.42P =0.49), postprandial blood sugar (kw=2.2 P =0.33), and glycosylated hemoglobin (kw=1.53 P =0.47) were not significantly different in the patients from three different altitudes.
The overall distribution of hemoglobin was significantly different (kw=68.9 P<0.001). Pairwise comparison of hemoglobin between the three groups showed that the values found in patients from Jinghong and Kunming (X2=41.7, P<0.001), Jinghong and Lijiang (X2=57.8, P <0.001) as well as Kunming and Lijiang (X2=12.2, P <0.001) were all significantly different.
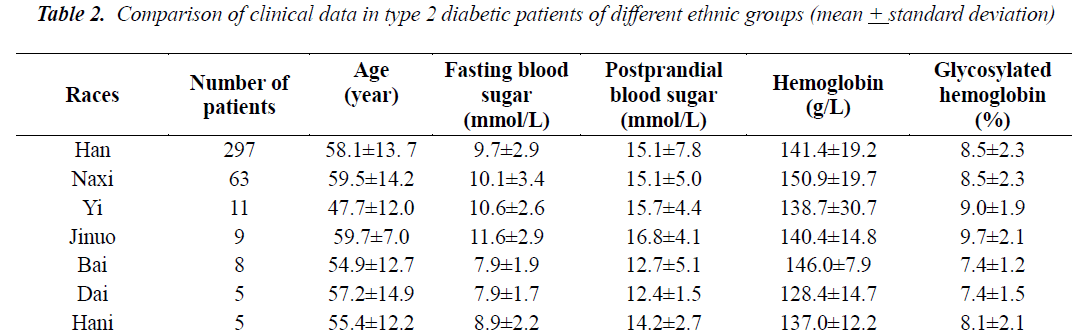
Table 2 compares the age difference, disease duration, fasting blood sugar, postprandial blood sugar, hemoglobin and glycosylated hemoglobin between the patients of 7 ethnic groups (Dai, Hani, Jinuo, Han, Naxi, Yi and Bai). There were more than 5 individuals from each ethnic group. The Kruskal-Wallis test was used for multiple independent samples. The overall distribution of age (kw=9.0, P =0.17), disease duration (kw=6.3, P =0.39), fasting blood sugar (kw=10.6, P =0.1), postprandial blood sugar (kw=6.0, P =0.4) and glycosylated hemoglobin (kw=7.5, P =0.28) was not significantly different in patients from various ethnic groups, while the overall distribution of hemoglobin was found to be significantly different (kw=15.8, P =0.015). Independent variables for correlation analysis of ethnic group to glycosylated hemoglobin showed that ethnic group did not have a significant effect on glycosylated hemoglobin (F=0.8, P =0.6).
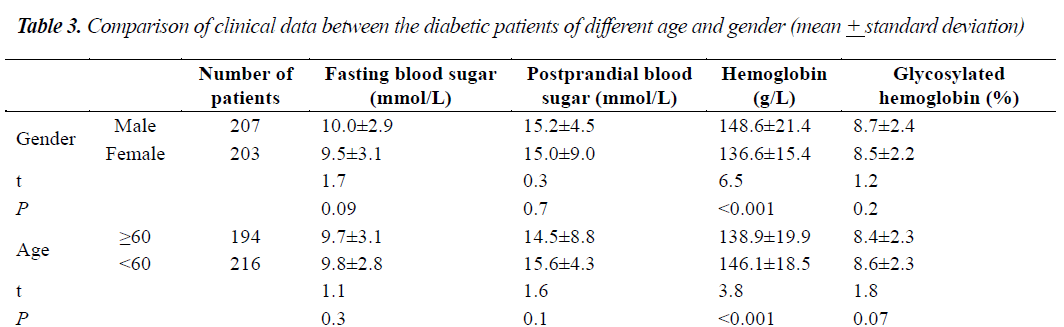
As shown in Table 3, the hemoglobin concentrations were significantly different between males and females, while the fasting blood glucose, postprandial blood glucose and glycosylated hemoglobin were not significantly different. Hemoglobin was significantly different between patients of 60 years old or above and patients below 60 years of age, while fasting blood glucose, postprandial blood glucose, and glycosylated hemoglobin were not significantly different between the patients of 60 years or above and patients below 60 years of age.
Discussion
Glycosylated hemoglobin is the product of continuous and irreversible non-enzymatic protein glycosylation reaction between hemoglobin (Hb) in red blood cells and glucose. HbA1C is the most stable glycosylated hemoglobin with highest content. Under conditions where the concentration of Hb is relatively stable, the level of glycosylation mainly depends on the blood glucose concentration and contact time between glucose and Hb [8]. The average life of red blood cells in circulation is about 120 days and the synthesis of HbA1c is a continuous process during the life of the red blood cells. Therefore, glycosylated hemoglobin levels reflect the patient’s average blood sugar levels over the 2-3 months period prior to the test. The American Diabetes Association first adopted HbA1c levels ≥6.5% as one of the four diagnostic criteria for diabetes in 2010; The WHO officially recommended HbA1c as the criterion for diagnosis of diabetes in 2011. At present, HbA1c is not only a monitoring index, but also a diagnostic index used in the United States, Europe, and Japan. China has launched the "Chinese glycosylated hemo globin education program" to promote the study of glycosylated hemoglobin [9] and accumulated data that will allow HbA1c to be used as a gold standard for diabetic blood sugar control as well as an index for diagnosis or screening of diabetes. Blood sugar is undoubtedly a main factor that affects levels of glycosylated hemoglobin, but other factors that may influence levels of glycosylated hemoglobin [10,11] include natural environments, age, gender and ethnicity. A complete understanding of the factors that affect HbA1c levels is important for clinicians, as it would allow them to accurately judge the patient’s control over his or her blood sugar levels, predict the risks of diabetes complications, and screen or diagnose diabetes.
Our findings showed that fasting blood sugar, 2-hour postprandial blood sugar, and glycosylated hemoglobin levels were not significantly different in the patients with type 2 diabetes from three different altitudes. There are several studies performed both in China and abroad on the influence of altitude on blood sugar and glycosylated hemoglobin levels. The research on Yang Lixinon healthy individuals showed that fasting blood sugar, 2-hour postprandial blood sugar, and glycosylated hemoglobin levels were not different from healthy adults of Suzhou (average elevation of 6m above sea level) and Xining (average elevation of 2260m above sea level). However, when these data were compared to individuals from Golmud (average altitude of 2800m above sea level), adults from Golmud showed similar levels of fasting blood sugar and 2-hour postprandial blood sugar as found in the adults from Suzhou and Xining, but showed different levels of glycosylated hemoglobin. Castillo et al. [12] found, that people from high altitude have significantly lower levels of fasting blood sugar and postprandial blood sugar than people from low altitude, but their fasting and postprandial insulin levels are not significantly different. Their study, however, did not include glycosylated hemoglobin. Baracco et al. found that fasting blood sugar levels at a high altitude population (4462m above sea level) were lower than those in a low altitude population (110m above sea level) [13].
A study by Sayarlioglu et al. showed that among patients with type 2 diabetes, blood sugar, glycosylated hemoglobin, and prevalence rate of retinopathy were not statistically different at high altitude (1727m above sea level) and at sea level [14]. Chen et al [15] showed that fasting blood sugar in healthy people was slightly increased on the 3rd and 25th day following an excursion from low altitude to an altitude of 2200-3800m, but their postprandial blood sugar decreased, suggesting that high altitude training may improve insulin sensitivity in healthy adults. Our study showed that hemoglobin in patients with type 2 diabetes is significantly higher in patients at high altitude compared to that at low altitude. Hemoglobin levels were statistically different between patients from three different altitudes and the concentration of hemoglobin increased with increasing altitude, suggesting that altitude has a significant effect on hemoglobin concentration. It is known that as a compensatory mechanism to the stimulus from chronic hypoxia at high altitude for an extended period of time, erythropoietin levels increased, subsequently red blood cells count and hemoglobin concentration also increase. The results of our study showed that after eliminating patients with hemoglobinopathy, hepatic and renal dysfunction and hemophthisis, which can be caused by a variety of reasons, the increase of hemoglobin does not affect patients’ fasting blood sugar, 2-hour postprandial blood sugar and glycosylated hemoglobin.
Yunnan is a multi-ethnic province. In our patient sample, five or more individuals represented seven ethnic groups. These are the Han, Naxi, Yi, Dai, Hani, Jinuo and Bai, which allowed us to analyze differences in serum levels of blood sugar and hemoglobin among these ethnic groups. Levels of blood sugar, postprandial blood sugar and glycosylated hemoglobin were not different between diabetic patients from different ethnic groups, but hemoglobin levels differed between the patients of different ethnic groups. This observed difference may be related to the different geographical environments inhabited by different ethnic groups. Herman et al. [16] showed that HbA1c was different in 0.15-0.4% of non-Caucasian patients with impaired glucose tolerance, such as those of Hispanic, Asian and African origin. Another study by Herman [17] compared average blood sugar, fasting blood sugar, postprandial blood sugar and glycosylated hemoglobin in more than 2000 patients of Caucasian, African, Spanish and Asian origin with diabetes and found that postprandial blood sugar in Asians was higher than in Caucasians, while fasting blood sugar and average blood sugar were not different between Asians and Caucasians. Fasting, postprandial, and average blood sugar levels were not different between Africans, Hispanics and Caucasians.
Glycosylated hemoglobin was not different between Caucasians and Africans, while it was slightly higher in Hispanics and Asians than in Caucasians and Africans. Hawkins [18] showed that glycosylated hemoglobin in healthy Asians is 0.3-0.6% higher than in Caucasians, and is highest in Indians, Malays, Eurasians, and Chinese, in descending order. Venkataraman et al. [19] showed that when fasting blood sugar is low, Malays and Indians have lower HbA1c than Chinese. When fasting blood sugar is over 5mmol/L, Malays and Indians have higher HbA1c than Chinese. The observed difference between Malays, Indians and Chinese increased with increasing fasting blood sugar. When fasting blood sugar was at the threshold for diagnosis of diabetes (7mmol/L), the contribution of ethnicity to glycosylated hemoglobin was 0.19-0.24%. Dynamic blood sugar monitoring of healthy people of different races by Hill showed [20] that average blood sugar is not different in Caucasians, Hispanics, Asians and Africans. The period during which patient blood sugar was lower than 3.5mmol/L and higher than 7mmol/L was 1.2% and 2.1%, respectively, and there was no difference between races. The above studies indicate that there is not a uniform conclusion as to whether race or ethnicity has an effect on blood sugar and glycosylated hemoglobin and whether the effect is clinically significant. Our results using variance analysis of ethnic group as a single factor for HbA1c using 13 ethnic groups as independent variables showed that different ethnic groups have little effect on glycosylated hemoglobin.
Our results showed that neither gender nor age has any significant influence on blood sugar and glycosylated hemoglobin, but age and gender do affect hemoglobin. A study of healthy people by Wu et al. [21] indicated that with increasing altitude, men had more hemoglobin than women among Han people, but not among Tibetan people. Above 2664m, this gender-related difference in hemoglobin concentration increased from childhood to young adulthood more significantly in Han people than in Tibetans, which indicate an effect of altitude, ethnic group, and gender on hemoglobin. As for the difference in glycosylated hemoglobin between genders, Herman [17] suggested that HbA1c is higher in females than that in males. However, Misra [22] showed that glycosylated hemoglobin is not significantly different between males and females, which is consistent with our findings.
References
- Duckworth W, Abraira C, Moritz T, et al. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Eng J Med 2009; 360: 129-139.
- Kim C, Herman WH, Cheung NW, Gunderson EP, Richardson C. Comparison of hemoglobin A1c with fasting plasma glucose and 2h post-challenge glucose for risk stratification among women with recent gestational diabetes mellitus. Diabetes Care 2011; 34: 1949-1951.
- Silverman RA, Thakker U, Ellman T, et al. Hemoglobin A1c as a screen for previously undiagnosed prediabetes and diabetes in an acute-care setting. Diabetes Care 2011; 34: 1908-1912.
- Sarwat S, Ilag LL, Carey MA, Shrom DS, Heine RJ. The relationship between self-monitored blood glucose values and glycated haemoglobin in insulintreated patients with Type 2 diabetes. Diabet Med 2010; 27: 589-592.
- Sattar N, Preiss D. HbA1c in type 2 diabetes diagnostic criteria: addressing the right questions to move the field forwards. Diabetologia 2012; 55: 1564-1567.
- Lin Y, Xu Y, Chen G, et al. Glycated hemoglobin, diabetes mellitus, and cardiovascular risk in a crosssectional study among She Chinese population. J Endocrinol Invest 2012; 35: 35-41.
- Yu Y, Ouyang XJ, Lou QL, et al. Validity of glycated hemoglobin in screening and diagnosing type 2 diabetes mellitus in Chinese subjects. Korean J Intern Med 2012; 27: 41-46.
- Schnedl WJ, Lahousen T, Krause R, et al. Evaluation of conditions associated with glycated hemoglobin values below the reference range. Clin Lab 2007; 53:179-181.
- Ji LN, Cai XL. Diagnosis of diabetes mellitus with glycated hemoglobin in China, a long way to go. Chin Med J (Engl) 2011; 124: 3605-3606.
- Nitin S. HbA1c and factors other than diabetes mellitus affecting it. Singapore Med J 2010; 51: 616-622.
- Grimsby JL, Porneala BC, Vassy JL, et al. Raceethnic differences in the association of genetic loci with HbA1c levels and mortality in U.S. adults: the third National Health and Nutrition ExaminationSurvey (NHANES III). BMC Med Genet 2012; 13:30.
- Castillo O, Woolcott OO, Gonzales E, et al. Residents at high altitude show a lower glucose profile than sea-level residents throughout 12-hour blood continuous monitoring. High Alt Med Biol 2007; 8: 307-311.
- Baracco R, Mohanna S, Seclen S. A comparison of the prevalence of metabolic syndrome and its components in high and low altitude populations in peru. Metab Syndr Relat Disord 2007; 5: 55-62.
- Sayarlioglu H, Erkoc R, et al. Nephropathy and retinopathy in type 2 diabetic patients living at moderately high altitude and sea level. Ren Fail 2005; 27: 67-71.
- Chen MT, Lee WC, Chen SC, et al. Effect of a prolonged altitude expedition on glucose tolerance and abdominal fatness. Res Q Exerc Sport 2010; 81: 472- 477.
- Herman WH, Ma Y, Uwaifo G, et al. Differences in A1c by race and ethnicity among patients with impaired glucose tolerance in the diabetes prevention program. Diabetes Care 2007; 30: 2453-2457.
- Herman WH, Dungan KM, et al. Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1, 5-anhydroglucitol in over 2000 patients with type 2 diabetes. J Clin Endocrinol Metab 2009;94: 1689-1694.
- Hawkins R. Differences in HbA1c between Caucasians, Chinese, Indians, Malays and Eurasians. Clin Chim Acta 2011; 412: 1167.
- Venkataraman K, Kao SL, Thai AC, et al. Ethnicity modifies the relation between fasting plasma glucose and HbA1c in Indians, Malays and Chinese. Diabet Med 2012; 29: 911-917.
- Hill NR, Oliver NS, Choudhary P, et al. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther 2011; 13: 921-928.
- Wu T, Wang X, Wei C, et al. Hemoglobin levels in Qinghai-Tibet: different effects of gender for Tibetans vs. Han. J Appl Physiol 2005; 98: 598-604.
- Misra R, Lager J. Ethnic and gender differences in psychosocial factors, glycemic control, and quality of life among adult type 2 diabetic patients. J Diabetes complications 2009; 23: 54-64.