- Biomedical Research (2011) Volume 22, Issue 3
Comparison between tris-citric acid yolk, yolk albumin citrate and skimmed milk extenders on sperm motility, livability and mass movement in frozen-thawed goat sperm.
Nor-Ashikin MNK1* and RBAbdullah21Institute of Medical Molecular Biotechnology, Faculty of Medicine, Universiti Teknologi MARA, Sungai Buloh Cam-pus, Jalan Hospital, 47000 Sungai Buloh, Selangor, Malaysia
2Animal Biotechnology-Embryo Laboratory (ABEL), Institute of Biological Sciences, Faculty of Science, University of Malaya 50603, Kuala Lumpur, Malaysia
- *Corresponding Author:
- Nor Ashikin Mohamed Noor Khan
Institute of Medical Molecular Biotechnology
Faculty of Medicine, Universiti Teknologi MARA
Sungai Buloh Campus, Jalan Hospital
47000 Sungai Buloh, Selangor
Malaysia
Accepted March 27 2011
Abstract
This experiment was carried out with the aim to compare the effect of three extenders on frozen-thawed sperm characteristics in goat. Sperm of Katjang and their crossbreds; Jer-masia and Jermana were cryopreserved in three extenders namely tris-citric acid yolk (TCAYE), yolk albumin citrate (YACE) and skimmed milk (SME) and the frozen-thawed sperm parameter were examined. The finding of this study showed that the Jermasia and Jermana buck sperm cryopreserved in TCAYE provided the best results in terms of motility (52.64 ± 0.93% and 53.20 ± 1.13% ) and livability (56.47± 0.96% and 57.72 ± 1.11%), re-spectively. In the crossbreds, sperm motility and livability in TCAYE were found to be sig-nificantly higher compared to the sperm cryopreserved in YACE and SME. Therefore, TCAYE extender apparently is better than YACE followed by SME for the cryopreserva-tion of goat sperm.
Keywords
Cryopreservation, Extender, Buck sperm
Introduction
Variation in the results of buck semen cryopreservation is attributed to many confounding factors which include processing techniques, choice of cryoprotectants, extend-ers dilution, cooling and thawing rates [1,2]. According to Purdy [1], an extender for cryopreservation serves as an energy source and protects sperm cells from cryogeni-cally-induced damage, thus maintaining sperm survivabil-ity. The choice of extender plays an important role in de-termining the viability of deep frozen semen. Different species have dissimilar nutritional requirements, and thus the type of extender used must cater for its specific needs. For example, the egg yolk-coagulating enzyme (EYCE) secreted by the bulbourethral gland of the goat has been shown to cause egg yolk coagulation and sperm death [3]. This phospholipase-A enzyme hydrolyses egg yolk leci-thin to fatty acids and lysolecithin [4,5]. Lysolecithin is toxic to goat sperm. A protein identified as SBUIII from the same gland, was also found to decrease survival of cooled or frozen goat sperm diluted in milk-based extend-ers [6]. The component that causes this effect was later identified as tricylglycerol lipase [7]. The component caused a decrease in sperm motility and the quality of movement in skim milk extender.
Materials and Methods
Experimental animals
Three local Katjang bucks and nine crossbred Jermasia and Jermana bucks ageing nine months to three years were used in this study. Animals for semen collection were selected based on the criteria suggested by Mukher-jee [8]. The crossbreds in the study were the products of a crossbreeding project jointly carried out between the In-stitute of Advanced Studies, University of Malaya, Ma-laysia and the Technical University of Berlin, Germany.
Local Katjang goats were inseminated with imported fro-zen semen from improved German Fawn bucks to obtain the F1 generation. The following generations, F2 to F5 goats were produced by inter-insemination procedure named Jermasia. Goats having 75% improved German Fawn genome and 25% Katjang goat genome were mated to improved German Fawn to upgrade them. The up-graded animals were then inter-inseminated to produce the Jermana genotype.
Collection of semen
Semen collection was done between 0800 to 0900 hr by using an artificial vagina (AV). The semen collection tube was wrapped by aluminum foil to minimise exposure to light. AV method required the use of restrained female goats in oestrus as teasers.
Preparation of extenders
Tris-citric acid yolk extender (TCAYE)
The TCAYE consisted of 3.8 g tris (hydroxymethyl) ami-nomethan, 2.1 g citric acid to make up to 100 mL with milli-Q water. After which, 0.05 g of streptomycin sulfate and 0.03 g penicillin-G (sodium salt) were added. Ratio of buffer to egg yolk was 4:1. After centrifugation at 650 x G for 15 min, 6.8% glycerol and 1.0% fructose were add-ed to the supernatant.
Yolk albumin citrate extender (YACE).
The YACE extender consisted of 2.9 g sodium citrate, 1.3 g D-glucose, 0.05 g streptomycin sulfate and 0.03 g peni-cillin-G (sodium salt) in 70 ml milli-Q water. Ratio of buffer to egg yolk was 4:1. After centrifugation at 650 x G for 15 min, 15 ml egg albumin and 6 ml glucose were added to 15 ml of supernatant.
Skimmed milk extender (SME)
A mixture containing 10.0 g skim milk (less than 1% fat content), 0.2 g D-glucose in 100 ml milli-Q water was heated at 91 ± 1ºC for 10 min, cooled to room tempera-ture and added with 0.03 g streptomycin sulfate and 0.02 g penicillin-G (sodium salt). Then 14% glycerol was add-ed to 86% of this mixture.
Cryopreservation
French-type 0.25 ml polyvinylchloride (PVC) straws were used in the packaging of semen for freezing. All semen-filled straws were left with an air space at its distal end and sealed with polyvinyl alcohol (PVA) powder. Straws were lined on a grooved perspex frame and kept in nitro- gen vapour (-150°C) for 9 min before plunging into liquid nitrogen.
Thawing
Frozen straw was taken out from liquid nitrogen and im-mersed in a water bath at 37°C for 2 min. All straws were wiped-dry before cutting off the tips and releasing cryo-preserved sperm into microfuge tubes in a floating water-bath rack at 37°C.
Evaluation of semen quality
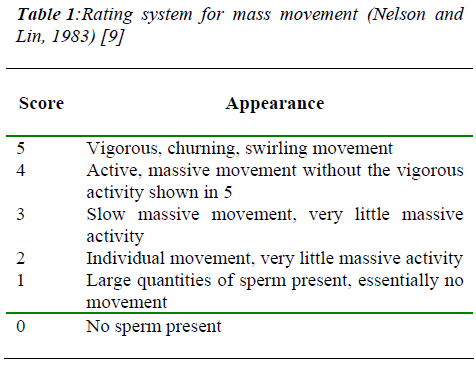
The quality of neat and frozen-thawed semen were evalu-ated based on composite characteristics. Percentage of sperm motility, livability and mass movement ratings (Nelson and Lin, 1983) [9] as shown in Table 1 were re-corded. Semen was diluted with basic tris-citric acid buf-fer (pH 6.75) prior to assess sperm motility.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) programme. Data were analysed through analysis of variance and Duncan’s Multiple Range Test. A P value of < 0.05 was considered to be statistically significant.
Results
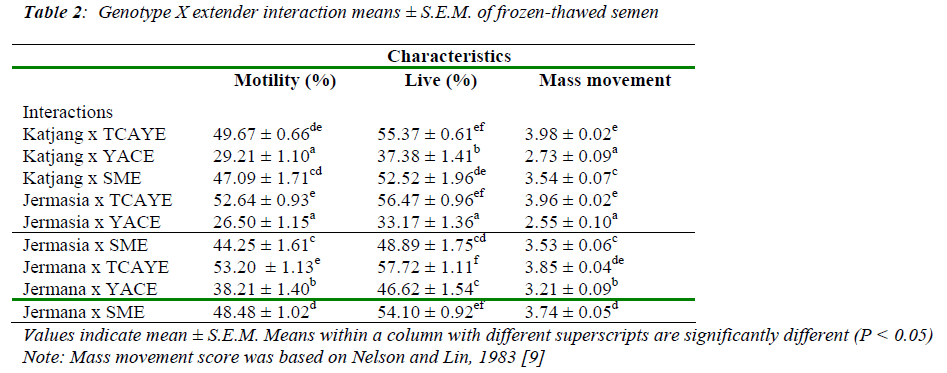
Table 2 shows results of post-thaw sperm characteristics of different buck genotypes in different extenders.
The finding of this study showed that the Jermasia and Jermana buck sperm cryopreserved in TCAYE provided the best results in terms of motility (52.64 ± 0.93% and 53.20 ± 1.13% ) and livability (56.47± 0.96% and 57.72 ± 1.11%), respectively. In the crossbreds, sperm motility and livability in TCAYE were found to be significantly higher compared to the sperm cryopreserved in YACE and SME.
Discussion
Interactions between Jermasia and Jermana bucks with TCAYE gave the best results in terms of motility (52.64 ± 0.93% and 53.20 ± 1.13% ) and live sperm percentage (56.47± 0.96% and 57.72 ± 1.11%), respectively. In the crossbreds, sperm motility and livability in TCAYE were significantly higher compared to sperm cryopreserved in YACE and SME. The mean values for motility and liv-ability sperm in all extenders were higher in Jermana bucks compared to Katjang and Jermasia bucks which is also reported by Noran [10]. The sperm motility and mass movement were found to be higher for crossbred goat. For all genotypes, TCAYE was found to be the best ex-tender in terms of frozen-thawed sperm motility, livability and mass movement. This finding is consistent with the finding of Dorado et al. [11], who found that tris extender produced better frozen-thawed sperm motility and veloc-ity parameters compared to skimmed milk extender, as measured by sperm class analyser (CASA). Hidalgo et al. [12] in their comparative study by using commercial tris- and skimmed milk-based extenders and found that milk extender caused less reduction of sperm head size in Flor-ida bucks. However, cryopreservation of Florida goat se-men using TCAYE extender results in slightly improved rate of motility (56.07 ± 1.32) [13]. Studies have shown that the removal of seminal plasma by washing immedi-ately after collection increases motility and livability of sperm during storage in egg yolk or milk extenders [14,15].
References
- Purdy PH. A review on goat sperm cryopreservation.Small Rumin Res. 2006; 63: 215-225.
- Medrano A, Terrazas A and Soto R. Principles and perspectives for the conservation of goat buck sper- matozoa. Small Rum Res 2010; 89: 140-143.
- Roy A. Egg yolk coagulating enzyme in the semen and Cowper’s gland of the goat. Nature 1957; 179: 318-319.
- Iritani A, Nishikawa Y. Studies on the egg-yolk coagu- lating factors in goat semen: II Properties of the coagu- lating factor and influential conditions for coagulation.In: Proc Silver Jubilee Lab Anim. Husbandry, KyotoUniversity; 1961 pp. 97-104.
- Iritani A, Nishikawa Y. Studies on the egg-coagulating enzyme in goat semen: IV. On the position of yolk con- stituents attacked by the coagulating enzyme. Jpn J Anim Reprod 1963; 8 (4): 113-117.
- Nunes JF, Corteel JM, Combarnous Y, Baril G. Rˆole du plasma s´eminal dans la survie in vitro des sper- matozo¨ıds de bouc. Reprod Nutr D´ev 1982; 22: 611-620.
- Pellicer MT. Purificacion y caracterizacion del componente de la secrecion bulbouretral de macho cabrio implicado en el deterioro de la calida de los spermatozoides diluidos en leche. In: Tesina de Licenciatura. Universidad de Murcia; 1995 pp. 200.
- Mukherjee TK. Collection of goat semen for A.I. in Artificial insemination of goats. 1988; Ed. G Deichert, S Sivaraj, T Banumathi and TK Mukherjee. Institute of Advanced Studies, University of Malaya, Kuala Lum- pur, pp 1-18.
- Nelson EA, Lin TY. Collection, evaluation, processing and storage of goat and sheep semen. Mimeographed notes, California State Polytechnic University 1983; pp19. Personal communication.
- Noran AM. Comparison between local Katjang and cross-bred bucks for semen characteristics and freez- ability, libido and body conformation. PhD thesis, Insti- tute of Advanced Studies, Universiti Malaya 1992.
- Dorado J, Rodríguez I, HidalgoM. Cryopreservation of goat spermatozoa: Comparison of two freezing extend- ers based on post-thaw sperm quality and fertility rates after artificial insemination. Theriogenology 2007; 68:168-177.
- Hidalgo M, Rodríguez I, Dorado JM. The effect of cryopreservation on sperm head morphometry in Flor- ida male goat related to sperm freezability. Anim Re- prod Sci 2007; 100: 61-72.
- Dorado J, Hidalgo M, Muñoz A, Rodríguez I. Assess- ment of goat semen freezability according to the sper- matozoa characteristics from fresh and frozen samples.Anim Reprod Sci 2009; 112: 150-157.
- Naing SW, Wahid H, Mohd Azam K, Rosnina Y, Zuki AB, Kazhal S, Bukar MM, Thein M, Kyaw T, San MM. Effect of sugars on characteriscs of Boer goat se- men after cryopreservation. Anim Reprod Sci 2010; 122: 23-28.
- Corteel JM. Viabilty of goat spermatozoa deep frozen with or without seminal plasma. Glucose effect. AnnBiol Anim Biophys Bioch 1974; 14: 741-745.
- Leboeuf B, Restall B, Salamon S. Production and stor- age of goat semen for artificial insemination. Anim Re- prod Sci 2000; 62: 113-141.
- van der Westhuysen JM. Observations on the deep freezing of Angora goat semen. S Afr J Anim Sci 1978; 8: 111-113.