- Biomedical Research (2013) Volume 24, Issue 4
Comparative study of marker of oxidative stress among normotensive, pre-hypertensive and hypertensive subjects.
Aquil Ahmad1, Mohd Mobark Hossain1, Usha Singhal3, Najmul Islam21Department. Of Physiology, JNMC, Aligarh Muslim University, Aligarh, UP, 202002, India
2Department of Biochemistry, JNMC, Aligarh Muslim University, Aligarh, UP, 202002, India
3Department. of Physiology, NIMS Medical college, Jaipur, Rajesthan, India
- Corresponding Author:
- Aquil Ahmad
Department of Physiology
Jawaharlal Nehru Medical College
Aligarh Muslim University
Aligarh, UP-202002, India
Accepted Date: June 01 2013
Citation: Ahmad A, Hossain MM, Singhal U, Islam N. Comparative study of marker of oxidative stress among normotensive, pre-hypertensive and hypertensive subjects. Biomedical Research 2013; 24 (4): 491-495.
Abstract
This study was undertaken to evaluate the serum levels of antioxidant enzymes and marker of lipid peroxidation and compare oxidative stress level among normotensive, prehypertensive and hypertensive subjects. In this cross sectional study 25 normotensive,15 prehypertensive and 25 hypertensive subjects were included. The potential participants were subjected to selection protocol consisting of clinical history, physical examination and appropriate test. All subjects underwent blood pressure measurement and markers of oxidative stress were estimated in serum. Exclusion criteria were smoking, diabetes, asthma, COPD, malignancies, chronic diseases, and current use of any medication including dietary supplements Present study showed strong association of oxidative stress with high blood pressure and there was positive correlation between marker of oxidative stress and systolic and diastolic blood pressure.
Keywords
Essential hypertension, Oxidative Stress, Oxidative Stress markers, Catalse, Glutathione peroxidise, Superoxide dismutase, Malondialdehyde
Introduction
Cardiovascular disease counted 2.3 million death in India in year 1990; this is predicted to double by the year 2020. Hypertension is directly responsible for 57% of all stroke death and 24% of all coronary heart disease in India [1]. Although it has frequently been indicated that the causes of essential hypertension are not known, this is only partially true. Essential hypertension is a heterogeneous disorder, with different patients having different causal factors that lead to high blood pressure.
Oxidative stress is described as a condition in which cellular antioxidant defenses are inadequate to completely inactivate the reactive oxygen species (ROS) and reactive nitrogen species (RNS) generated because of their excessive production , loss of antioxidant defenses, or both [2]. Vascular oxidative stress has been demonstrated in spontaneous (genetic) and experimental models of hypertension [3,4]. Studies using nonspecific markers of oxidative damage have observed higher superoxide and hydrogen peroxide production in hypertensive subjects, which returned to levels observed for control subjects after blood pressure reduction [5]. Russo et al. [6] showed that essential hypertension is associated with greater than normal lipoperoxidation and an imbalance in antioxidant status, suggesting that oxidative stress is important in the pathogenesis of essential hypertension or in arterial damage related to essential hypertension. However, no difference was found in levels of some antioxidants between hypertensive patients and normal control subjects by Tse, Maxwell, Thomason, Blann, Thorpe, Waite, Holder R [7]. Similarly Ward et al. [8] have recently demonstrated no difference in either plasma or 24-h urinary F2-isoprostanes in treated or untreated hypertensive subjects compared with normotensive control subjects. So the role of oxidative stress in hypertension has become debatable.
This study was conducted with the following objectives:
1.Evaluate and compare the level of antioxidant en zymes namely Superoxide Dismutase(SOD), Catalase and Glutathione Peroxidase (GPX) between normotensive, prehypertensive and hypertensive subjects.
2. Evaluate and compare the level Malondialdehyde (MDA) which is marker of lipid peroxidation, between normotensive, prehypertensive and hypertensive subjects.
3. To determine correlation between between antioxidant enzymes and blood pressure.
4. To determine correlation between between MDA and blood pressure.
5. To determine the relationship between blood pressure and oxidative stress and association of oxidative stress with hypertension.
Material and Method
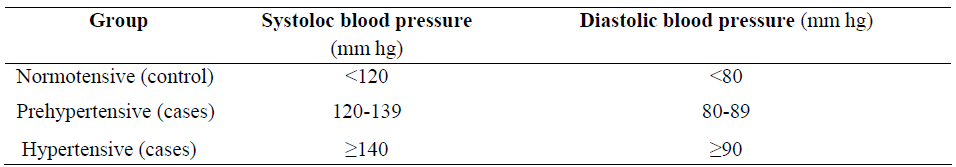
A cross-sectional design was applied to 25 healthy normotensive, 15 prehypertensive and 25 hypertensive subjects. On the basis of blood pressure level following groups were made according to JNC-VII classification [9].
Subjects included in this study were between 30-60 years. Control group included normotensive subjects with blood pressure of ≤120/80 mm Hg while cases included those with blood pressure of >120/80 mm Hg. Case group were subdivided in two category (i) Prehypertensive group with systolic blood pressure of 120-139 mm Hg and diastolic blood pressure of 80-89 mm Hg. (ii) Hypertensive group with systolic blood pressure of ≥140 mm Hg and diastolic blood pressure of ≥90 mm Hg. Cases group include only untreated essential hypertensive subject. Exclusion criteria were smoking, diabetes, asthma, COPD, malignancies, chronic diseases, and current use of any medication including dietary supplements. The potential participants were subjected to selection protocol consisting of clinical history, physical examination and appropriate test. Blood pressure was recorded by auscultatory method by using sphygmomanometer on regular working day in left arm in sitting position.
Under aseptic conditions and with prior consent of subject, 5ml of blood samples was drawn from peripheral vein and was centrifuged at 3000 rpm for fifteen minutes. The serum was subjected to estimation of Malondialdehye (MDA) which is a marker of lipid peroxidation and antioxidant enzymes namely Catalase, Glutathione peroxidise (GPX), Superoxide Dismutase(SOD) by prior adopted method [10,11,12,13]. Protein content of serum was estimated by the method put forth by Lowry [14]. Antioxidant enzymes were expressed with respect to serum protein level.
The study protocol was approved by ethics committee of institution. All participants signed a written consent form and no complications was encountered during the study.
Statistical analysis
Statistical analysis was done using statistical package for social science (SPSS 17) . The results were expressed as Mean±Standard Deviation (SD). Differences between the groups was analysed using one way ANOVA with the post hoc Tukey test. Pearson’s correlation was applied to determine the relationships between variables. Statistical significance was defined at P<0.05.
Result
Clinical characteristics and serum level of markers of oxidative stress in normotensive, prehypertensive and hypertensive groups are shown in Table-I. Anti-oxidant enzymes (SOD, Catalase, GPX) were significantly decreased in prehypertensive and hypertensive group when compared with normotensive group. Similarly Antioxidant enzymes level was significantly decreased in hypertensive group when compared to prehypertensive group. MDA level was significantly increased in hypertensive group when compared with normotensive group but there was no significant difference in MDA level between prehypertensive and normotensive group(p>0.05). MDA level was significantly increased in hypertensive group as compared to prehypertensive group as per Table II.
Data presented are mean ± SD. Analysis of data was done by one-way ANOVA and post-hoc by Tuky test. The * depicts comparison with normotensive and the #depicts comparison with prehypertensive. ** P < 0.05; ##P < 0.05.
Table 2: Comparison of Marker of Oxidative Stress Between Normotensive, Prehypertensive and Hypertensive Subjects.
Table III, and Table iv. shows that there was negative correlation between antioxidant enzymes and systolic and diastolic blood pressure.MDA level showed positive correlation with systolic and diastolic blood pressure. This shows that there is positive correlation between oxidative stress and level of blood pressures
Discussion
ROS play a physiological role in vessel wall and participate as second messengers in endothelium dependent function in smooth muscle and endothelium cell growth and survival and in remodeling of vessel wall. Each of these response, when uncontrolled contribute to vascular disease (Irani et al-2000, Taniyama et al-2003)[15,16]. Free radicals have diverse roles in the vascular redox systems in the patients of hypertension, thus the complexity of the redox signaling in the distinct spatial spectrums should be considered for a better understanding of the hypertension. Our study demonstrated decrease in antioxidant enzymes in prehypertensive group and hypertensive group when compared with control group.There was increase in MDA level in hypertensive group when compared with normtensive group. Also antioxidant enzymes showed negative correlation with systolic and diastolic blood pressure .Similarly MDA showed positive correlation with systolic and diastolic blood pressure.Thus it showed that oxidative stress is associated with hypertension and there is positive correlation between oxidative stress and blood pressure.
The explanation for reduced SOD activity is possible due direct inactivation of enzymes by hydrogen peroxide or by the superoxide anion itself (Salon DC et al-1998, Sinet PM et al-1981)[17,18]. The decrease in Catalase activity in our study may be attributed to its inactivation as a result of continuous exposure to hydrogen peroxide and hydrogen peroxynitrite. The decrease can also be due to down regulation of its gene expression (Simic DV et al- 2006)[19]. Pedro-Batet J et al-2000[20] observed reduction in SOD and GPX activity in newly diagnosed untreated hypertensive compared with control subject with SOD activity being inversely correlated with blood pressure with the hypertensive group but not in control subject. Radon J et al-2003[21] found that activity of antioxidant enzymes were significantly lower in whole blood from hypertensive patients compared with those of normotensive subjects.
Cracowski JL[22] et al-2003 found that in never-treated mild-to-moderate hypertension, lipid peroxidation was not increased suggesting that ROS may not be critical in the early stages of human hypertension, but could be more important in severe hypertension which is consistant with present study. Nwanjo HU et al-2007[23] reported increase in plasma MDA level, which is marker of lipid peroxidation due to oxidative stress. Similarly Parmer RJ et al-2000[24] also reported higher production of hydrogen peroxide in treated and untreated hypertensive subjects compared with normotensive subjects with a significant correlation between hydrogen peroxide level and systolic blood pressure.They above mentioned studies support our observation that there is association of oxidative stress and hypertension and there is positive correlation between serum level of marker of oxidative stress and high blood pressure.
It has become clear that virtually all cells in the vessel wall (endothelial, smooth muscle and adventitial cells) produce ROS in varying amount and in response to diverse stimuli, which can act in an autocrine or paracrine fashion to modulate cellular function (Griendling et al, 2000) [25]. The major vascular ROS is superoxide anion (.O2ˉ), Which inactivate nitric oxide(NO), the main vascular relaxing factors, thus impairing relaxation (Cai & Harrison et al-2000)[26]. Also ROS causes depletion of tetrahydrobiopterin(BH4), an important NO synthase cofactor( Vaziri ND et al-2000)[27]. Dismutation of .O2ˉ by Superoxide Dismutase (SOD) produce hydrogen peroxide (H2O2), a more stable ROS, which in turn is converted to water by Catalase and Glutathione peroxidase. H2O2 and other peroxidase appear to be important in the regulation of the growth-related signaling in vascular smooth muscle cells and inflammatory response in vascular lesion (Li PF et al, 1997)[28]. ROS causes structural and functional alteration by several ways, including direct damage to endothelium cell eicosanoid metabolism, altered redox state, increase in intracellular free calcium concentration and stimulation of inflammatory and growth signaling events(Zalba G et al-2001, Chen X et al-2001, Schiffrin EL et al-2002)[29,30,31]. Thus oxidative stress promotes vascular smooth cell proliferation and hypertrophy and collagen deposition leading to thickening of vascular media and narrowing of vascular lumen. Oxygen radicals may also induce endothelial permeability with extravasation of plasma protein and macromolecules and recuruitment of inflammatory proteins and cells, which could further impair endothelial function and aggravate vascular damage. All these effects on vasculature may explain how oxidative stress can cause hypertension.
The major limitation of our study was the small sample size.Financial constraints were the main cause of choosing a small sample size. Multi centric studies with larger numbers of subjects are required to extrapolate these results to the general population.
References
- Gupta R. Trends in hypertension epidemiology in India. J.Human Hypertension 2004; 18: 73-78.
- Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis [Review]. Physiol Re.v 2004; 84: 1381-478
- Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y: Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension 2004; 43: 841-848.
- Tanito M, Nakamura H, Kwon YW, Teratani A, Masutani H, Shioji K, Kishimoto C, Ohira A, Horie R, Yodoi J: Enhanced oxidative stress and impaired thioredoxin expression in spontaneously hypertensive rats. Antioxid Redox Signal 2004; 6: 89-97.
- Kumar KV, Das UN. Are free radicals involved in pathobiology of human essential hypertension?Free Radic Res Commun1993; 19: 59-66.
- Russo C, Olivieri O, Girelli D, Guarini P, Carletto A, Corrocher R: Anti-oxidant status and lipid peroxidation in patients with essential hypertension.J Hypertens 1998; 16: 1267-1271.
- Tse WY, Maxwell SR, Thomason H, Blann A, Thorpe GH, Waite M, Holder R: Antioxidant status in controlled and uncontrolled hypertension and its relationship to endothelial damage. J Hum Hypertens 1994; 8: 843-849.
- Ward NC, Hodgson JM, Puddey IB, Mori TA, Beilin LJ, Croft KD: Oxidative stress in human hypertension: association with antihypertensive treatment, gender, nutrition, and lifestyle. Free Radic Biol Med 2004; 36: 226-232.
- Chobanian AV, Bakris GL, Black HR et al. Seventh report of the Joint National Committee on revention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension.2003; 42 (6). 1206–52.
- Philpot J. Assay for MDA levels. Rad Res.1963; 3: 55- 80.
- Aebi HE. Catalase in vitro. Meth Enzymol.1984; 105: 121-126.
- Paglia DE, Valentine WN. Studies on the quantitative characterization of erythrocyte glutathione peroxidise. J lab Clin Med 1967; 70: 158-168.
- Mc Cord JM, Fridovich I. Superoxide dismutase an enzymic function for erythrocuprein hemocuprein. J Biol Chem 1969; 244: 6049-6055.
- Lowry OH, Rosebrough NJ, Farr AL. Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193: 265-275.
- Irani K. Oxidant signalling in vascular cell growth, death and survival: A review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signalling. Circ Res 2000; 3: 179-183.
- Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanism. Hypertension 2003; 6: 1075-1081.
- Salon DC, Lin SW, Pacific RE, Davies KJ. Superoxide dismutase is preferentially degraded by proteolytic system from RBC following oxidative modification by hydrogen peroxide. Free Radic Biol Med 1998; 5: 335- 339.
- Sinet PM, Garber P. Inactivation of human copper zince SOD during exposure to superoxide anion and hydrogen peroxide. Arch Biochem Biophys 1981; 212: 411-416.
- Simic DV, Mimic-oka J, Pljesa-Ercegovac M, Savic- Radojevic A, Opacic M, Matic D. By products of oxidative protein damage and antioxidant enzymes activites in plasma of patients with different degrees of essential hypertension. J Hum Hypertens 2006; 20: 149-155.
- Pedro-Batet J, Covas MI, Martin S, Rubies-Prot J. Decreased endogenous anti oxidant enzymatic status in essential hypertension. J Hum Hypertens 2000; 14: 343-345.
- Radon J, Oliva MR, Tarmas C, Giner V, Choves J, Iradia A, Saez GT. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension 2003; 41: 1096-1101.
- Cracowski JL, Baguet JP, Ormezzano O, Bessard J, Stanke-Labesque F,Bessard G, Mallion JM. Lipid peroxidation is not increased in patients with untreated mild-to-moderate hypertension. Hypertension 2003; 41 286-288.
- Nwanjo HU, Oze.G, Okafor MC, Nwasu D, Nwankpa P. Oxidative stress and non enzymic antioxidant status in hypertensive patients in Nigeria. African journal of Biotechnology 2007; 6: 1681-1684.
- Parmer RJ, Lacy F, Kailasam MT, O’Connor DT, Schmid-Schonbein GW. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender and ethinicity. Hypertension 2000; 36: 878-884.
- Griendling KK, Harrison DG. Dual role of reactive oxygen species in vascular growth Circ Res 2000; 6: 562-563.
- Cai, Harrison DG. Endothelial dysfunction in cardiovascular disease: The role of oxidative stress. Circ Res.2000;10:840-844.
- Varizi ND,Ni Z, Oveisi F, Tranasky-Hobbs DL.Effect of antioxidant therapy on blood pressure and NO synthase expression in hypertensive rats. Hypertension 2000; 36: 957-964.
- Li PF, Dietz R, Von Hardon R. Differential effect of hydrogen peroxide and superoxide anion on apoptosis and proliferation of vascular smooth muscle cells. Circulation1997; 10: 3602-3609.
- Zalba G, Sanjose G, Mareno MU, Fortuno MA. Oxidative stress in arterial hypertension.Role of NADPH oxidase.Hypertension2001; 6: 1395-1399.
- Chen X, Touyz RM, Park JB, Schiffrin EL.Antioxidant effects of Vit C and Vit E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke prone SHR. Hypertension 2001; 38: 606-611.
- Schiffrin EL.Beyond blood pressure:The endothelium and atherosclerosis progression.Am J hypertens 2002; 15: 1155-1225.



