Research Article - Journal of Agricultural Science and Botany (2018) Volume 2, Issue 1
Comparative efficacy of carp pituitary extract and Ovaprim during captive breeding of Cat Fish, Pabda (Ompok pabda).
Nihar Ranjan Chattopadhyay*
Department of Biotechnology, Govt. of India, Rajiv Gandhi University, Rono Hills, Doimukh, Itanagar 721119, Arunachal Pradesh, India
- *Corresponding Author:
- Nihar Ranjan Chattopadhyay
Visiting Research Professor
Department of Biotechnology
Govt. of India, Rajiv Gandhi University
Arunachal Pradesh, India
E-mail: nrchatterjee40@gmail.com
Accepted March 09, 2018
Citation: Chattopadhyay NR. Comparative efficacy of carp pituitary extract and Ovaprim during captive breeding of Cat Fish, Pabda (Ompok pabda). J Agric Sci Bot. 2018;2(1):25-36.
DOI: 10.35841/2591-7897.2.1.25-36
Visit for more related articles at Journal of Agricultural Science and BotanyAbstract
Captive breeding was conducted to compare the efficacy of CPE and Ovaprim on O. pabda and it was observed that both the inducing agents were quite effective. However, R3 (CPE) dose (5 mg/kg bd.wt. to male and 16 mg/kg bd.wt. to female) registered less fertilization and hatchlings compared to the T3 dose of Ovaprim (0.5 ml/kg bd.wt. to male and 1.5 ml/kg bd.wt. to female). As compared to the conventional hypopysation, which requires high quantity of carp pituitary extract (CPE), Ovaprim is quite effective at low quantity for inducing breeding under captivity. Vijaya kumar reported induce spawning of O. malabaricus with single dose Ovaprim 0.5 ml/ kg bw while Banik et al. reported induce spawning of O. bimaculatus with single dose Ovaprim 0.5 ml/kg bd.wt. of male and 1.5 ml/kg bd.wt. to female. Haniffa et al. advocated three different dosage of Ovaprim (0.3 ml, 0.5 ml and 0.7 ml) for induce breeding of O. malabaricus where the variations in fertilization rate were 70.87 and 91% and survival no. and rate was 58.62 and 70% respectively. Three different treatments, during the experiment ensures a high rate of fertilization (66.88 & 65.40) and hatching (78.40%), when highest survival rate was 59.8, 62.1 and 61.4% respectively with three treatments of Ovaprim.
The results of the experimental data indicate that, for successful spawning and seed production of Pabda, Ovaprim can be a better option than Carp pituitary extract. It is envisaged from the data that Ovaprim not only induce complete spawning but fertilization, hatching and survival is more ensured than CPE. Further study should be conducted for better management and understanding about the reproductive physiology, breeding behavior and larval rearing of Pabda to save this unique fresh water species.
Keywords
CPC, Ovaprim, Latency period, Captive breeding, Fertilization, Hatching.
Introduction
Ompok pabda, popularly known as “Butter cat fish”, is a freshwater species native to India, Bangladesh, Pakistan, and Myanmar. This species is an indigenous catfish belongs to the family Siluridae of the order Siluriformes. It is listed as endangered fish species [1-4]. The fish has a wide geographical distribution covering Afghanistan, Bangladesh, Myanmar, the Indus plain and adjoining hill area of Pakistan, the North East States of India particularly in Bihar and West Bengal during the early 1970s. Due to rich lipo-protein content and soft bony structure this fish species is considered delicious and nutritious to the people of East India, North East India and Bangladesh as well [5-8].
This species occurs in the lotic and fresh water ecosystem [1,9]. Also available in beels/mauns connected with rivers. In nature the fish breeds in rivers, reservoirs and water sheds, while in flooded condition during south west monsoon. However, in the early 1980s sharp decline in occurrence and abundance were observed [10,11]. Despite its greater economic value this species did not receive sufficient attention in aquaculture. Meager occurrence of live samples in nature and poor survival of the larvae are major constrains of the observations [12]. As a result of indiscriminate fishing during monsoon, application of pesticide in agriculture, soil erosion and siltation formation in river, contamination of habit due to sewage and industrial pollution etc. The population of this species is sharply reduced [13,14].
The catfish belongs to the genus Ompok (La Cepede, 1803), mainly consists of medium-sized silurid fishes found in inland waters throughout South and Southeast Asia. The genus is traditionally diagnosed by the presence of a short dorsal fin with 4 rays, strongly forked caudal fin, subcutaneous eye, set immediately posterior to the mouth rectus and two patches of palatal teeth [15]. Born Busch showed that Ompok, as currently understood, is probably paraphyletic [16], distinguished by relatively short maxillary barbells (not extending beyond tip of pectoral fin) and 48-54 branched anal-fin rays. Males are slenderer and have strong serrations on the posterior edge of the pectoral spine (females lack these serrations). Ompok pabda is highly priced, delicious, good taste, fine flesh with soft meat texture and high nutritional value and well preferred fish as possess relatively few bones. This species is silvery in color with two one at distinguished spots, shoulder and another close to the base of tail; longitudinal stripes of dark color often present on the sides of the body. This species which attains a length of 24 cm is caught in fairly large numbers and as a nourishing food fish especially for invalids; it fetches a very high price. The price is above Rs. 300/-per kg (US $1= Rs.52/-).
The species is recently prioritized as a new candidate species for freshwater aquaculture [17,18]. It is piscivorous as well as carnivorous and prefers to inhabit lakes, ponds and rivers. In India, It is widely distributed in the plains and sub mountainousregions. over the last decade, its wild population has undergone a steady decline (>50%), mainly due to over exploitation, loss of habitat, disease, pollution, siltation, poisoning, dynamiting, indiscriminate fishing during the breeding season, wide use of pesticide and insecticides from agriculture fields and other destructive fishing operations and changes in hydrology of the rivers due to human intervention.
Thus, Ompok pabda has been categorized as an endangered (EN) fish species according to IUCN based CAMP report [4]. It is necessary to develop captive breeding program and culture techniques for these endangered species, in order to save them from extinction. It has not been received much attention in aquaculture mainly due to non-availability of information regarding its breeding and culture technique. However, successes have been achieved in breeding and seed rearing of Pabda in India. The Regional Research Centre of Central Institute of Freshwater Aquaculture, Kalyani, West Bengal has been working on the subject for the past five years to develop and standardize the breeding and seed production techniques of this fish. Recently this Center has successfully achieved mass breeding and seed production of Pabda, after several trials.
In natural habitat, pabda achieves maturity at the end of first year of growth. The fecundity is as high as 8,400 to 13,800 nos/100 g of gravid fish weight. Since the availability of pabda seed in nature is poor and the procurement of seed is difficult, induced spawning has been adopted for its widespread propagation as well as large scale production of seed for extensive farming practices. Proper care and maintenance of brood fish is the primary step for successful breeding. Generally, pabda weighing 40 g and above (male and female) are considered as suitable for breeding. The sexes can be easily identified by examining the secondary sexual characters they develop during the breeding season. The abdomen of the gravid female is soft and bulged and genital papilla is fleshy and large in size. While in mature males the genital papilla is conical in shape. Mature fishes are generally stocked and raised up to the gravid stage in earthen ponds (0.01-0.04 ha). Lime applied at the of 500 kg/ha and the pond is manured with cattle dung 10 t/ha. In the brood stock pond, water hyacinth and hydrilla are grown to simulate the natural condition of their habitat. These plants provide as ideal shelter for the fishes and also serve as a source of periphytic food organisms. The depth of the pond was maintained between 1.0- 1.5 m. The brood fishes are fed daily with a compounded feed of rice bran, oil cake and fish meal (1:1:0.5) at 5% of average body weight of fishes under farming.
Artificial Breeding
The breeding season of O. pabda extends from May-August. The brood fishes of 35-40 g are usually selected for inducing them to breed successfully. Fishes of both sexes were induced by using either carp pituitary extract or the branded commercial product “Ovaprim”. The doses of Ovaprim varied at 1-1.5 ml/ kg of female and male 0.5 ml/kg of male. The injected fishes were stripped after 10-15 hrs. By gently squeezing the abdomen towards the tail. The eggs were collected in clean dry plastic/ enamel/steel tray. A good female may yield ripe eggs to the extent of about 15-20% of its body weight. The abdomens of males were cut open and testes were removed carefully. They were finely chopped and macerated to prepare sperm suspension. The collected milt was then added to eggs and thoroughly mixed using a feather. The eggs were cleaned by washing in freshwater and transferred to a flow- through system or a fiberglass tank with aeration facilities for incubation. The fertilized eggs were spread uniformly in a plastic tub or fiberglass tank with a water depth of 5 cm. A feeble current of water was created, having the support of an aeration facility. In about 30 minutes of fertilization of eggs, the blastodisc was formed. The yolk plug stage was attained in 5 hours. There was enclosed. A slight thickening of one end of the embryo indicated cephalic region. The notochord was formed rudimentarily in 8 hrs. and the embryo acquired a kidney shape. In 18 hrs, the caudal region appeared distinctly. The gut was faintly indicated, in a position posterior to the yolk sac. The egg hatched out in 24 hrs. The newly hatched larvae were cylindrical in shape. They were transparent with a pale greenish yolk sac, visible prominently. The yolk sac got absorbed in 3 days. After hatching, the larvae were collected by siphoning them out from the bottom of the hatching tank. The yolked larvae measured about 4-5 mm in length, 4-7 days old spawn were fed with finely sieved zooplankton in alternate days and later with chopped tubifex worms. The fry attained 5-6 cm/3-4.5 g in a rearing period of 40-50 days with a survival of 80%. Fingerlings of this size are considered fit for stocking in grow-out farming ponds.
Breeding of Pabda is important, so as to stock them in natural habitats for restoring the gene bank of this endangered fish. Seed production and culture technologies also need to be developed for such non- conventional fish as Snakehead (Shoal and taki), Climbing Perch (Koi) Catfish (Boal, Air, Rita) and Eel (Baim). Ompok pabda fetch a good price in the market due to its palatability and nutritive value. Considering its economic importance and especially in the perspective of its rapidly declining population, efforts have been initiated to standardize the breeding and larval rearing of this unique species. Trial has been conducted by both the State Government as well as Kolkata Centre of CIFA. All the trials were conducted using Ovaprim as inducing agent, with not so satisfactory results.
Materials and Methods
Ompok pabda, popularly known as butter fish, belongs to the family Siluridae, is important for customer choice and fetches a high market value. Natural abundance of Pabda is becoming decreased by days due to environmental degradation and destruction of breeding ground. Entire breeding program including brood stock management as well as nursery management was conducted in private hatchery.
Recently the species has been prioritized as new candidate species for freshwater aquaculture, when IUCN has declared it as endangered one [19]. As of now various experiments were being conducted to protect this species from being extinct. Standardization of breeding protocol and mass production of seed of this endangered fish species are important to save this unique species. The methodologies adopted are the following -
Locality of the study area
The study was conducted in a private hatchery in West Bengal, India- one of the leading hatchery in West Bengal and well known for innovative Breeding Program.
Brood stock collection and management
Adult male of the size group 15.2-17.5 cm and female of 17.2- 19.8 cm were collected from River during November- February andwere stocked in pond (0.5 ha). The breeding and larval rearing were carried out at the same farm. A monthly water exchange of 50% was made to maintain water quality parameters within favorable ranges. Proper management practices were initiated for rapid enhancement of growth and maturity of the brooders. Aquatic macrophytes like Hydrilla verticillata and Eichhornia crassipes etc. were inoculated as these plants serve as ideal shelter for the fishes and also asa source of periphytic food organisms. The depth of the pond was maintained between 1.0- 1.5 m. After 1-2 month of rearing the fishes attained maturity and were considered for breeding.
Feed and fertilizer management
For brooders: The fishes were fed with supplementary feed made up of mustard oil cake, rice bran (1:1 ratio), chopped earthworms, small live prawns and small fishes like Puntius sp. at a 5% of average body weight per day. Added to this, fishes were fed also with trash fish (Bombay duck), boiled chicken viscera and boiled gram (Figure 1) at about 5-10% of total body weight per day. Trash fish/boiled chicken viscera kept hanging (3 inch under water from surface level) by thread at an interval of two inch. Fishes were reared for a period of 6 months and feeding schedule was maintained throughout the culture period. The fish attained a body weight of 30-45 g.
For spawn: 24 hrs. after hatching the spawns were fed with grinded/ chopped tubifex worms at morning (7 am) and evening (4-5 pm) for two days (Figure 2). Again some grinded tubifex worms were given for two days; then again light grinded worms were given to hatchlings for 7 days still they attain half inch size. The fry attained 5-6 cm/3-4 5 g in a rearing period of 40- 50 days with a survival of 72.4%. Fingerlings of this size are considered fit for stocking in grow-out ponds. Recommended feeding schedule is maintained both for broodstock and nursery management in farms with monthly fertilization. Just before the start of the breeding season a change, in feeding schedule, is made from February onwards and the brooders were fed with boiled pea/chicken viscera. Just after the onset of breeding season the feed is given regularly to spent brooders to enhance gonadal recrudescence.
Management of water quality
The physico-chemical characteristics of water of the brooders pond, brooder tank and hatching tank were recorded periodically during the period of one year. The variations in water parameters such as temperatures, pH, dissolved oxygen, alkalinity and hardness were estimated following standard methods [20]. Three different readings were recorded for each tank (Table 1).
| Pond | Temp (°c) | pH | DO (ppm) | Alkalinity (ppm) | Hardness (ppm) |
|---|---|---|---|---|---|
| Brooders pond | 29 ± 1 | 7.5 – 7.8 | 6 – 6.5 | 130 ± 10 | 110 ± 15 |
| Breeding tank | 31 ± 1 | 7.6 – 7.9 | 7 – 7.6 | 140 ± 15 | 112 ± 10 |
| Incubation tank | 29 ± 1 | 7.5 – 8 | 6.9 – 7.2 | 135 ± 15 | 107 ± 15 |
Table 1. Water Quality parameters.
The depth of water was recorded by a measuring tape.
Breeding season and maturation
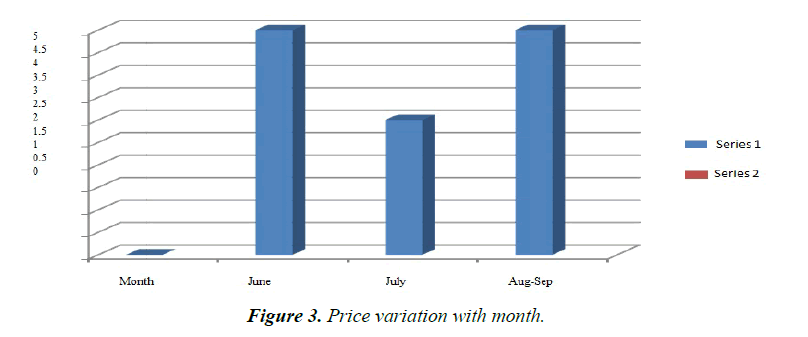
The fish attained maturity at the end of the first year. Males matured earlier than female. Maturity cycle of the species was studied byexamination of gonads at different months. During November-January the fishes were found to be in stage I and II of maturity. While they attained III stageof maturity in March. Majority of the males were found to be matured during late March-April, while the bulk of females were only in stage-IV. Fully ripe females were observed during May to the end of July. Breeding season extends from early June to late August, sometime extends up to September. The fry price varies with the season, during early days in June the price is Rs.5.00/ fry, which slowly begins to decline to Rs.4.00/fry as the season reaches its peak during July (monsoon). Again, on and from last part of July and up to August-September, again there is a price hike (Table 2 and Figure 3). The reason for such variations is due to non-availability of brood fish at the start and end of season.
| Sl No. | Month | Price (Rs.)/fry |
|---|---|---|
| 1. | June | 4.00-5.00 |
| 2. | July | 3.00-4.00 |
| 3. | Aug-Sept | 5.00 - 6.00 |
Table 2. Price variation with month.
Breeding practice
Selection of breeders: During breeding season (June-August), fully ripe females and males were identified by their secondary sexual character. Males are comb like and slightly swollen abdomenfor developing milt within, but normally it doesn’tooze out on slight pressure to abdomen. Besides males possess an elongated and pointed genital papilla. In females, the abdomen is soft and bulging for providing space to grown up on shaped hard roes inside, vent reddishwith rounded genital papilla. The pectoral fin spine, relatively longer and thicker in male than female, and more prominent.
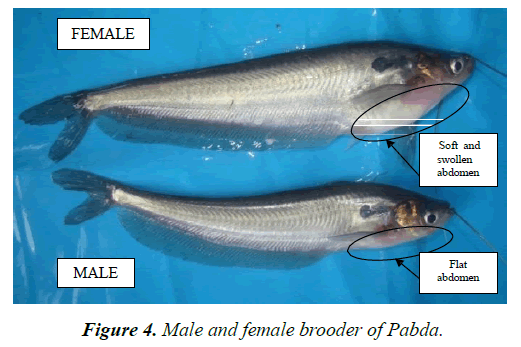
Identification of male and female fish: As in other fish dorsal surface of the pectoral fin is rough and comparatively large than female, genital papillae is pointed and whitish milt come out during peak of maturity phase, but in female pectoral fin is small and smooth. Abdomen swollen for holding eggs and at maturity individual eggs come out through pinkish vent on slight pressure to abdomen (Figure 4).
Age and size of brooders: As reported Chakraborty et al., [21] fish attained maturity at the end of the first year and male mature earlier than females, which become mature at 30-40 gm weight. Banik et al. reported that maturity cycle of Ompok bimaculatus in first year [2]. Mature female’s spawns on induction and get fertilized with sperm when mixed with sperm suspension. Ovaprim was used at different dosages ranging from 27°C-32°C. Hatching time has been recorded for 23-24 hrs. After fertilization at about average fertilization rate is being 72%. This species matures during April-May in Bengal and Assam and have a fairly extended breeding season till the end of July. Sarkar, et al. reported that three years old Pangasius is more responsive to captive breeding (Figure 5) [22].
Sex ratio: The average sex ratio considered for breeding in captivityis found to be 9:6=M:F (Tables 3-6). It is observed that three fully matured and healthy males are sufficient to fertilize the eggs of two or more numbers of female. Moreover, there is a dire scarcity of mature and sufficient number of healthy brooders in the hatcheries. For this the farmers often, inadvertently, use the skewed sex ratio for achieving the targeted production, not aware of the consequences.
| Treatment (Tank) |
Mean ± SD Male( cm) |
Mean ± SD Female (cm) |
Dose of Hormone (ml/kg. bd.wt.) | |||
|---|---|---|---|---|---|---|
| Length | Weight | Length | Weight | Male | Female | |
| R1 | 17.14 ± 0.23 | 28.08 ± 1.00 | 18.80 ± 0.47 | 34.88 ± 1.13 | 3 | 12 |
| R2 | 17.02 ± 0.20 | 29.11 ± 0.80 | 19.23 ± 0.36 | 35.33 ± 0.33 | 4 | 14 |
| R3 | 17.39 ± 0.37 | 28.72 ± 0.67 | 19.12 ± 0.19 | 35.40 ± 0.32 | 5 | 16 |
Table 3. CPE protocol used during experiment.
| Treatment (Tank) |
Mean ± SD (cm) Male | Mean ± SD (cm) Female | Dose of Hormone (ml/kg. bd.wt.) | |||
|---|---|---|---|---|---|---|
| Length | Weight | Length | Weight | Male | Female | |
| T1 | 16.83 ± 0.69 | 28.85 ± 1.73 | 18.81 ± 0.70 | 35.28 ± 2.72 | 0.5 | 1 |
| T2 | 16.42 ± 0.27 | 28.26 ± 1.79 | 18.88 ± 0.89 | 35.18 ± 3.44 | 0.5 | 1.2 |
| T3 | 16.47 ± 0.22 | 28.24 ± 1.75 | 18.90 ± 0.96 | 37.35 ± 2.40 | 0.5 | 1.5 |
Table 4. Ovaprim protocol used in the experiment.
| Hormone | Treatment | ||
|---|---|---|---|
| CPE | R1 | R2 | R3 |
| Ovaprim | T1 | T2 | T3 |
Table 5. Expt. Set up for CPE and Ovaprim.
| Treatment (Experiment tank) |
Sex Ratio | Dose of CPE hormone (mg/kg bw) |
||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| R1 | 9 | 6 | 3 | 12 |
| R2 | 9 | 6 | 4 | 14 |
| R3 | 9 | 6 | 5 | 16 |
Table 6. Sex Ratio and different doses of “CPE” in three experiment tanks.
Restocking of brooders: Restocking of brooders after spawning is a common practice adopted by hatchery owner. The hatchery owners stock the brooders and kept as potential brood stock for the ensuing season and the subsequent years.
Inducing agent: Mainly two types of inducing agents are used for induce breeding of fish. One is the natural induce agent called Carp Pituitary Extract (CPE) (Figures 6 and 7) and other is synthetic inducing agent like Ovaprim (Plate H). The hatchery owners of Bengal still use CPE as the sole inducing agent, as the same are easy available at a rate of Rs.2-3 and may be due to organic in origin. But due to uncertainty in procuring the pituitary gland and for some advantages, now a day, pituitary is being replaced by Ovaprim. At present 80% of the farmers are using Ovaprim for induce breeding program against 20% of CPE users.
CPE and Ovaprim protocol used in the experiment: During experiment, pituitary protocol as followed for each experimental tank is given in (Table 3).
It was observed that pre-monsoon and post-monsoon breeding required higher dose, may be due to non-availability of congenial environmental condition. Ovaprim protocols are presented in (Table 4).
Experimental design
Two different hormones i.e. CPE and Ovaprim are used as inducing agent at varying doses. Total six experiments were conducted both for CPE (3 sets, which is denoted by R1, R2 and R3) and Ovaprim (3 sets, which was denoted by T1, T2 and T3).
All experiments were conducted in the well maintained hatchery for a period of one month (Table 5). At first all brooders were collected from the stocking pond and are stocked into two hapa (135 cm x 90 cm x 130 cm) fixed at the pond five hours before injection. The ratio was 9:6 (M: F) as given Table 6. The experimental set up for both the inducing agents are presented in (Table 5).
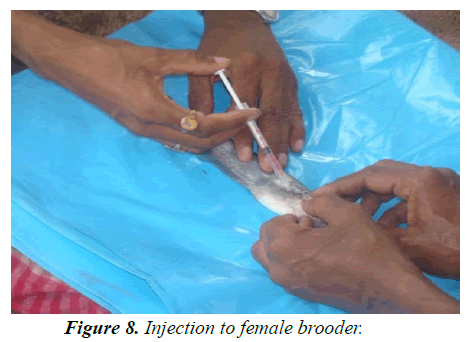
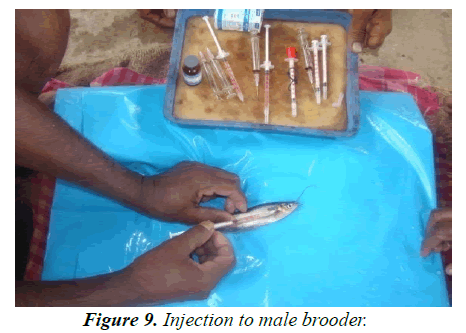
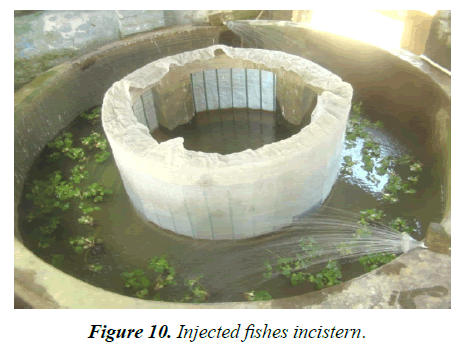
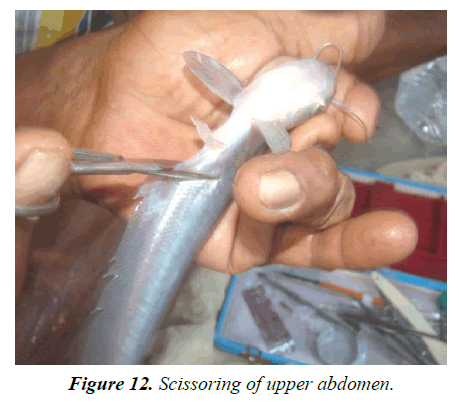
Experiment tank-R1: According to size, fishes (average length 17.5-18.27 cm; weight 33.5 gm-36 gm) were injected accordingly. Single dose was provided 12 mg/kg body weight for female and 3 mg/kg body weight for male 7 AM (Figures 8 and 9). Both injected fish were then maintained inbreeding tank (cistern) with Eichhornia (act as stimulating agent) for mating for about 7 hrs at the ratio of 9 males:6 females (Figure 10). Slow flow rate was maintained and the eggs were checked at interval for ovulatory response, after sometime sex wise segregated fishes were kept in hundis (Figure 11), maintaining water temperature at 29°C and hardness at 110 ppm. After 9 hrs, injected females were stripped (Figures 12-15). After stripping the female were released into the stocking pond giving dip treatment in Potassium permanganate solution of 0.1 ppm and in Tetracycline hydrochloride (water soluble antibiotic, Intervet India Pvt. Ltd., Thane) to prevent any infection and for speedy recover from stress condition. Dose depends upon the intensity of the infection/stress. The eggs are collected in a clean dry enamel/steel/glass tray. The abdomens of induced males are dissected to remove the testes (Figures 12 and 13) kept in Petri dish with 0.9% normal saline water (Figure 14). Testes are then thoroughly macerated with the help of very fine nylon net (Figure 16) and sperm suspensions are prepared with 0.9% saline solution (Figure 17). Sperm suspensions are then distributed over the eggs and mixed gently with a feather for through mixing and fertilization (Figure 18). After 3-5 minutes clean water is added and washed thoroughly for complete removal of excess milt. After a while, they were washed thoroughly with distilled water. The eggs were light brown in color and swollen after water absorption (Figure 19). These are fertilized eggs which are then distributed by hand over the khancha (iron frame), kept in pre-arranged in incubation tank with provision of aerator (Figure 20). In the incubation tank, initially 4-inch water depth is maintained and after hatching, it was increased to 6 inch and maintained till 10-15 days with continuous aeration. Water quality parameters of the tanks are recorded regularly (Table 4) following the method given by APHA [20]. After 18 hrs fertilized eggs were hatched out. After hatching is completed the Iron frame (khancha) and the unfertilized eggs are removed carefully from the incubation tank. The tank is washed thoroughly and filled with the fresh water up to 6 inch with Hydrilla verticillata. At this stage the hatchlings had a tendency to gather at the margin of the tank. The hatchlings are very active and moved very fast showing schooling behavior and showed a tendency of lateral alignment due to yolk content on ventral side. The newly hatched larva possesses large yolk sac which is being absorbed by 3 days. After 3 days, barbells became prominent and mouth begins to develop and the larva starts accepting external feed. Initially hatchlings fed on egg yolk. After yolk sac absorption, grinded egg mixed with chopped tubifex is provided to each tank. After 10 days, Hydrilla is removed and in its place palm leaves are kept on the water surface for providing shelter to grown up hatchlings up to 15 days. The hatchlings now are ready to stock into the well-prepared nursery pond for further development (Figure 21-23).
Experiment tank-R2: Fishes (average length-16.5-17.83 cm; weight-33.3-35.58 gm) were injected single dose of 14 mg/kg body weight to female and 4 mg/kg body weight to male which at 7 AM. After induction the fishes are then maintained into a round cement tank for 7 hrs. at the ratio of 9:6 M:F (Figures 10 and 23). Slow flow rate was maintained. Before 9 hrs., eggs are checked at interval by small hand net for adulatory response. Water temperature maintained at 31°C and hardness at 112 ppm. Water quality parameters of the tanks are recorded regularly (Table 4). Same management procedure adopted as in R1.
Experiment tank-R3: Fishes (Average length-16.8-18.06 cm; weight-34.035-36.16 gm) were injected accordingly with a dose of 16 mg/kg body weight to female and 5 mg/kg body weight to male (Figure 8 and Table 7). All the other management practices adopted like R1.
| Treatment | Sex Ratio | Dose of Ovaprim hormone (ml/kg bw) |
||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| T1 | 9 | 6 | 0.5 | 1 |
| T2 | 9 | 6 | 0.5 | 1.2 |
| T3 | 9 | 6 | 0.5 | 1.5 |
Table 7. Different doses of “Ovaprim” in three experiments.
Experiment tank no-T1: According to size, fishes (Average length- 16.8-18.8 cm; weight- 28.85-35.28 gm) were injected with a single dose of 1 mg/kg body weight for female and 0.5 mg/kg body weight for male 7 AM (Figure 8). Both injected fish are then kept into a round cement tank (cistern) with Eichhornia crassipes for mating for 7 hrs at the ratio of 9 : 6= M: F (Figure 8). Slow flow rate is maintained and eggs were checked at interval by small hand net for ovulatory response. Water temperature maintained at 29°C and hardness at 107 ppm. After 9 hrs., injected females were stripped and the stripped female were released into the stocking pond giving dip treatment in Potassium permanganate solution of 0.1 ppm and in Tetracycline hydrochloride (water soluble antibiotic, Internet India Pvt. Ltd., Thane) to prevent any infection and for speedy recover from stress condition. Dose depends upon the intensity of the infection/stress. Stripped eggs are collected in a clean dry enamel/steel/glass tray. The abdomens of induced males are dissected to remove the testes and are thoroughly macerated with the help of very fine nylon net to extract the milt. A sperm suspension is prepared by mixing the milt with 0.9% saline solution. Sperm suspensions are then poured over the eggs and mixed gently with a feather for through mixing and fertilization. After 3-5 minutes clean water is added and washed thoroughly for complete removal of excess milt. After a while, they were washed thoroughly with distilled water. The eggs were light brown in color, and swollen after water absorption. These are fertilized eggs which are distributed by hand on khancha, kept pre-arranged in incubation tank with minimum provision of aerator. In the incubation tank, initially 4-inch water depth is maintained and after hatching it increased to 6 inch and maintained till 10-15 days with continuous aeration. Water quality parameters of the tanks are recorded regularly (Table 4) following APHA [20]. As hatching completed the Iron frame (khancha) and the unfertilized eggs are removed carefully from the incubation tank. The tank is washed thoroughly and filled with the fresh water up to 6 inch with Hydrilla verticillata. At this stage the hatchlings had a tendency to gather at the margin of the tank. The hatchlings are very active and moved very fast showing schooling behavior and showed a tendency of lateral alignment due to yolk content on ventral side. The newly hatched larva possesses large yolk sac which is being absorbed by 3 days. After 3 days, barbells became prominent and mouth begins to develop and the larva starts accepting external feed.
Initially hatchlings fed on egg yolk. As feed one grinded egg mixed with chopped tubifex is provided to each tank. After 10 days, Hydrilla is removed and in its place palm leaves is kept on the water surface for providing shelter to grown up hatchlings up to 15 days (Figure 21) The hatchlings now are ready to stock into the well-prepared nursery pond for further development.
Experiment tank-T2: According to size, fishes (average length-16.4 cm -18.8 cm; weight-28.26 gm-35.2 gm) were injected accordingly. Single dose of 1.2 mg/kg body weight for female and 0.5 mg/kg body weight for male was administered generally at 7 AM (Figure 8). Same management practices adopted as above (Table 4).
Experiment tank-T3: According to size, fishes (average length-16.5 cm -18.9 cm; weight-28.24 gm - 37.35 gm) were injected 1.5 mg/kg body weight for female and 0.5 mg/kg body weight for male 7 AM (Figure 8). Both injected fish are then kept into a round cement tank (cistern) with Eichhornia for mating for 7 hrs. at the ratio of 9:6 = M:F (Figure 10). Same management practice adopted as above.
Results
Effect of CPE and Ovaprim on Latency period
The mode of fish breeding operation was closely observed to study the latency period by using Carp Pituitary Extract and Ovaprim. The latency period recorded with CPE was 18 hrs. in R1, 17 hrs in R2, 16 hrs in R3 while it was 12 hrs. In T1, 11 hrs. In T2, 9 hrs in T3 with Ovaprim (Tables 8-10). This indicates Ovaprim require less latency period for all the three treatments.
| Hormone | Treatment | No. of eggs released | No. of fertilized eggs |
|---|---|---|---|
| CPE | R1 | 68765 | 51230 |
| R2 | 79577 | 60876 | |
| R3 | 84475 | 67748 | |
| Ovaprim | T1 | 64355 | 52771 |
| T2 | 88760 | 4114 | |
| T3 | 108565 | 93691 |
Table 8. Number of eggs release and fertilized with CPE and Ovaprim.
| Hormone | Treatment | No. of Hatchlings |
|---|---|---|
| CPE | R1 | 26229 |
| R2 | 34699 | |
| R3 | 42003 | |
| Ovaprim | T1 | 35251 |
| T2 | 48470 | |
| T3 | 73453 |
Table 9. Number of hatchlings with CPE and Ovaprim.
| Hormone CPE | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experiment Tank | No.of individuals (M:F) | Male L (cm) | Male wt (g) | Female L (cm) | Femal wt (g) | Dose of hormone (mg/ml) in Male | Dose of hormone (mg/ml)in Female | Latency period (hrs) | No. of eggs released | Fertilization (%) | No. of Fertilized eggs | Hatching (%) | No. of hatchlings | Survival rate (%) |
| R1 | M-9, F-6 |
17.14 ± 0.23 | 28.0 ± 1 | 18.80 ± 0.47 | 34.88 ± 1.13 | 3 | 12 | 18 | 68765 | 74.5 | 51230 | 51.2 | 26229 | 58.6 |
| R2 | M-9, F-6 |
17.02 ± 0.2 | 29.11 ± 0.8 | 19.23 ± 0.36 | 35.33 ± 0.33 | 4 | 14 | 17 | 79577 | 76.5 | 76.5 | 57 | 34699 | 61.2 |
| R3 | M-9, F-6 |
17.39 ± 0.37 | 28.72 ± 0.67 | 19.12 ± 0.19 | 35.40 ± 0.32 | 5 | 16 | 16 | 84475 | 80.2 | 67748 | 62.2 | 42003 | 61.6 |
| Hormone Ovaprim | ||||||||||||||
| T1 | M-9, F-6 |
16.83 ± 0.69 | 28.85 ± 1.73 | 18.81 ± 0.7 | 35.28 ± 2.72 | 0.5 | 1ml | 12 | 64355 | 82 | 52771 | 66.8 | 35251 | 59.8 |
| T2 | M-9, F-6 |
16.42 ± 0.27 | 28.26 ± 1.79 | 18.88 ± 0.89 | 35.18 ± 3.44 | 0.5 | 1.2ml | 11 | 88760 | 83.5 | 74114 | 65.4 | 48470 | 62.1 |
| T3 | M-9, F-6 |
16.47 ± 0.22 | 28.24 ± 1.75 | 18.90 ± 0.96 | 37.35 ± 2.4 | 0.5 | 1.5ml | 9 | 108565 | 86.3 | 93691 | 78.4 | 73453 | 61.4 |
Table 10. Comparative efficiency of CPE and Ovaprim on different breeding.
Number of eggs released and fertilized with CPE and Ovaprim
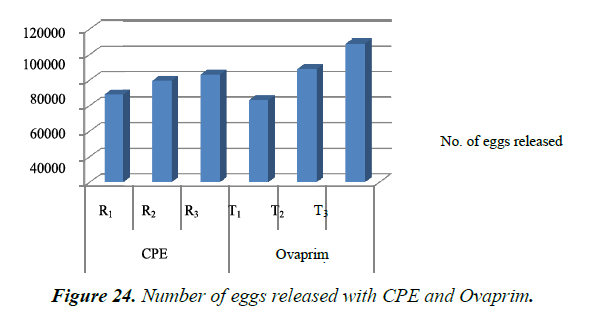
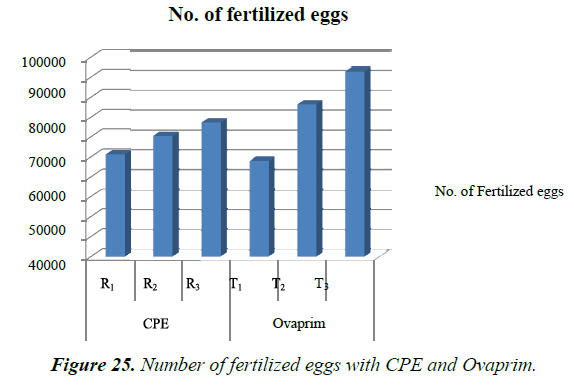
Number of eggs released and the eggs fertilized with CPE and Ovaprim during the study was recorded and compared (Table 8). It was found that number of eggs released with the Ovaprim (T3- 0.5 ml / kg. body weight in male and 1.5 ml/kg body weight in female) was higher (108565 numbers eggs) than the CPE (R3- 5 mg/kg. body weight in male and 16 mg/kg body weight in female) (84475 numbers eggs). Maximum number of fertilized eggs (93691 numbers) were with the Ovaprim, which was higher than the CPE (67748 fertilized eggs) (Figure 24 and 25).
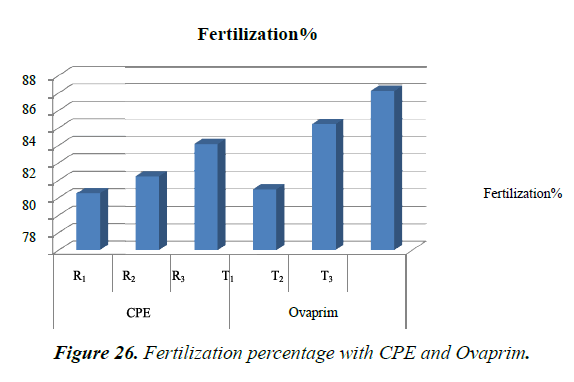
Highest numbers of hatchling (73453 hatchlings) were obtained at T3, in which male and female are treated with single dose of Ovaprim at the rate of 0.5 ml/kg and 1.5 ml/kg body weight respectively. Relatively lesser numbers of hatchings were obtained at T1 (3521) and T2 (48470). With CPE highest numbers of hatchlings were obtained at R3 (42003) followed by R2 (34699) and R1 (26229) (Table 9). Highest numbers of hatchling (73453 hatchlings) were obtained at T3, in which male and female are treated with single dose of Ovaprim at the rate of 0.5 ml/kg and 1.5 ml/kg body weight respectively. Relatively lesser numbers of hatchings were obtained at T1 (3521) and T2 (48470). With CPE highest numbers of hatchlings were obtained at R3 (42003) followed by R2 (34699) and R1 (26229) (Figure 26).
Survivality
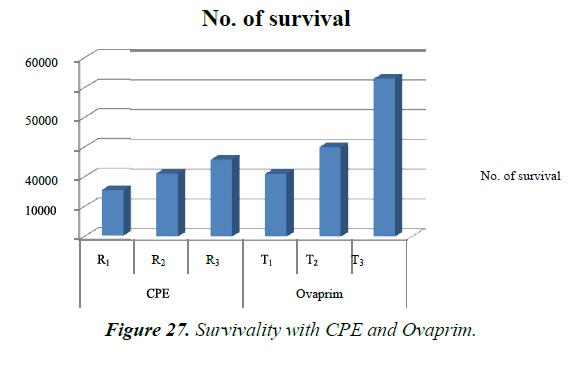
Survivality was maximum with T3, followed by T2, R3 and R1, while it was same for T1 and R2 but in both case the rate was more than R1.
Discussion
Realizing steadily declining trend in natural population, efforts were being initiated by Government of West Bengal, in collaboration with leading private hatcheries, to standardize the culture and propagation of Pabda in captive condition. Efforts are also initiated to establish gene banks using cryopreservation techniques. We have conducted this breeding trial in a leading private hatchery of west Bengal. A major breakthrough for captive breeding of O. pabda was achieved in 1999 [23] when some scientists of ICAR achieved partial success towards captive breeding in a private hatchery. In 2002, first success for commercial production of seed was achieved by a private hatchery in West Bengal, India. Our study indicate that the fish attain maturity at 1 year + (10-11 months) and male mature earlier than female. Females mature during May-July [24] and may extend from mid-April to mid-August [2]. Males mature first during late March-April. Fry price fluctuate during the breeding season with a moderate rate of Rs. 4.00-5.00 in May-June (pre monsoon), Rs.3.00-4.00 in July (monsoon) and Rs.5.00-6.00 in August-September (post monsoon). Compare to Bangladesh (Rs. 2.00/piece) the rate is high in Bengal (Table 2 and Figure 3). For breeding operation, 1 year+ broods with average wt. and size group of 28.24-37.35 g, and 16.4-18.9 cm respectively were considered for breeding operation with a skewed ratio of 1.5:1 (M: F) yielded higher no. of eggs compared to 1:1 ratio used by some other authors (Khanam, 2005). Stripping, as it ensures maximum fertilization, was undertaken during the experiment. With CPE best results were obtained at R1 and R2 treatment. Maximum spawning and fertilization were obtained when females were induced with an initial dose of 2-3 mg PG/ kg body weight followed by a resolving dose of 4-6 mg PG/kg body weight at an interval of 6 hrs. Mukherjee et al. advocated a single dose of 16 mg / kg BW for female with a yield of 4,200 eggs and hatching percentage of 50% [25]. Egg yield and fertilization was significantly higher in our tria, compared to previous experimental results. The Ovaprim protocols used for each treatment are presented in (Table 8). It was observed that the dose did not vary much with the long breeding season as observed with the pituitary gland (Figure 27).
The latency period, considered an important criteria for evaluation of inducing agent, varied with treatment as recorded in (Table 10), highest in R1 (18 hr) and lowest in R3 (16 hr) in respect of CPE. With Ovaprim it was highest with T1 (12) and lowest with T3 (9 hr). Latency period is reported to fluctuate depending on various factors such as temperature, pH, hardness and preparation of hormone etc. Chakrabarty et al. reported a 23 hr latency p