Research Article - Biomedical Research (2017) Volume 28, Issue 11
Comparative analysis of echocardiographic parameters between cerebral apoplexy and healthy controls
Yan Zhu, Zhibin Wang*, Rong Zhang, Xiuxiu Fu, Yuanyuan Meng, Rong Li and Dongmei Sun
Department of Cardiac Ultrasound, Affiliated Hospital of Qingdao University, Qingdao, Shandong Province, PR China
- *Corresponding Author:
- Zhibin Wang
Department of Cardiac Ultrasound
Affiliated Hospital of Qingdao University, P. R. China
Accepted date: April 10, 2017
Abstract
Aorta eddy is one of the most important parameters of aorta. The incidence of arteriosclerosis can affect the characteristics of eddy current. In this study, we applied trans-esophageal echocardiography to compare aortic vortex parameters between cerebral apoplexy patients (n=102) and normal controls (n=20). Cardiac ultrasound (time resolution=15.4 ± 3.7 milliseconds, 60-80 frames/cardiac cycle) was carried out through esophagus between two groups. Qualitative and quantitative analyses of aorta vortex were performed for the position, form, undulate shape and other parameters. In the control group, irregular-shaped vortexes were observed in the descending aorta position. The vortex of patients with cerebral apoplexy was in the single form, and a majority of them were round and partially merged, etc. In quantitative analysis of the depth of eddy current, the relevant parameters in the control group were significantly higher than those in the cerebral apoplexy group (all P<0.05). The incidence of aortic plaque in the cerebral apoplexy group was 56.4%, significantly higher than 10.9% in the control group (P<0.05). The Vortex Depth (VD) which reflected the main parameters of aortic vortex position in the control group was 0.612 ± 0.171, significantly higher compared with 0.582 ± 0.122 in the cerebral apoplexy (P<0.05). The eddy strength-related parameters of cerebral apoplexy patients were significantly higher than those in the control group. Statistical significances were identified between the cerebral apoplexy and control groups. Trans-esophageal echocardiography can effectively evaluate the form and intensive parameters of aorta vortex.
Keywords
Aorta vortex, Cerebral apoplexy, Trans-esophageal echocardiography
Introduction
Aorta is the largest artery in the human body. Thoracic aortic stiffness is correlated with cerebral apoplexy and other cardiovascular events. It is widely recognized that atherosclerosis is characterized with the hardening and thickening of the arterial wall. The artery wall contains lipid plaque deposition, which results from a variety of pathological factors including lipid metabolism abnormality, genetic susceptibility, hypertension, etc. The shear stress exerts the highest effect upon the hemodynamics of vascular endothelial cells [1-5]. Thoracic aorta possesses the characteristics of deuterostrophies, retrograde and eddy currents because of the complex anatomical structures, which is also the key determinant factor of hemodynamics [6,7]. The corresponding hemodynamic model tends to show abnormal changes when the lesions form. Computational fluid mechanics is commonly used to assess the fluid characteristics of the thoracic aorta, which is relatively better than the cardiovascular MRI because it can provide relatively comprehensive information on aortic hemodynamics by 3D visual flow pattern. However, it is limited by the restricted assumed model, time and space [8]. The application of Particle Image Velocimetry (PIV) for cardiac ultrasound can provide visualization and assessment of complex blood flow and eddy currents in vivo. Previous studies demonstrated that it exerts a comparative effect on the magnetic resonance in the evaluation of common carotid artery velocity [9-11].
Therefore, the aim of this study was to identify the difference of aortic vortex-related parameters in the patients diagnosed with cerebral apoplexy and their healthy counterparts by using the technique of trans-esophagus ultrasound, which contributes to the clinical diagnosis and treatment of cerebral apoplexy for physicians.
Materials and Methods
Baseline data
The study period was between August 1, 2014 and July 31, 2015, this study was a prospective study. The enrolled subjects were divided into the cerebral apoplexy (n=102) and control groups (n=20). Inclusion criteria: The diagnosis of cerebral apoplexy was validated by imaging tools including Computed Tomography (CT), Doppler ultrasound and MRI, etc. Exclusion criteria: Those patients were excluded if they were complicated with severe cardiac valve diseases, such as aortic regurgitation, stenosis, aortic aneurysm, severe heart rate disorders or atrial fibrillation, etc. Written informed consents were obtained from all participants. The study procedures were approved by the ethics committee of our hospital.
Ultrasound examination of the esophagus
American Acuson Sequoia C-512 ultrasonic platform and 7 MHz phased array transducer were utilized for ultrasound examination. After routine heart examination, the short-axis transverse section showed proximal segment of the thoracic aorta approximately 5 cm below the arcus aortae. The reagent mixture containing 0.7 ml Definity (Lantheus Medical Imaging, American), ultrasonic enhancement reagent (containing all fluorine propane gas) and 25 ml physiological saline was injected via the forearm vein. The contrast ultrasound image in the mechanical index dynamic range of 0.4-0.6, 60-65 dB was obtained and showed the highest possible frame rate by setting up the image depth, local position and other parameters. Three cycles of data by sound acquisition technology were performed at a time resolution of (15.4 ± 3.7) milliseconds and 60-80 frames per cardiac cycle.
Ultrasonic PIV
The obtained images were analysed by two imaging professionals who were blind to the study. The velocity of eddy current and quantitative analysis of form, position and phase variation of vortex were assessed by Omega flow PIV software (version 2.4.5, USA) and inter-group analysis was carried out. The frontal and posterior positions of the vortex center were determined based on the depth of eddy current, and expressed as decimal digit 0 and 1. Lateral position of the Vortex (VT) showed the inner-lateral position of the vortex center. Vortex Length (VL) and Vortex Width (VW) were used to determine the shape of the vortex. VL was divided by VW, and then the Spherical Index (SI) of vortex was calculated. The pulsation of the aorta vortex was evaluated by measuring the Relative Strength (RS) and Vortex Relative Strength (VRS).
Statistical analysis
All data analysis was performed by SPSS 20.0 software (SPSS Inc., Chicago, USA). Continuous variables were expressed as mean ± standard deviation. Comparison between two groups was analysed by using t-test. The ratio comparison was conducted by using chi-square test. A P value of less than 0.05 was considered as statistical significance.
Results
Baseline data
The mean age of cerebral apoplexy patients was (67.0 ± 12.0) years old, significantly older compared with (45 ± 9) years old in the control group (P<0.05). In both groups, the percentage of male subjects was significantly higher compared with that of female counterparts (P<0.05). The incidence rate of hypertension, diabetes mellitus and dyslipidemia in the cerebral apoplexy group was 71.2%, 33.5% and 13.2%, respectively. In the control group, no medical history of such complicated diseases was reported. The incidence of aortic plaque in the cerebral apoplexy group was 56.4%, significantly higher compared with 10.9% in the control group (P<0.05). There was no significant difference in the left ventricular ejection fraction, left ventricular mass index, left ventricular end diastolic diameter, left atrium volume index and EO rate between the two groups (all P>0.05). In the control group, the EO rate was 0.11 ± 0.04, significantly higher compared with 0.06 ± 0.05 in the cerebral apoplexy group (P<0.05). In the cerebral apoplexy group, the E/E rate was 12.1 ± 4.7, significantly higher compared with 6.9 ± 3.1 in the control group (P<0.05).
Apparent characteristics of the aorta vortex
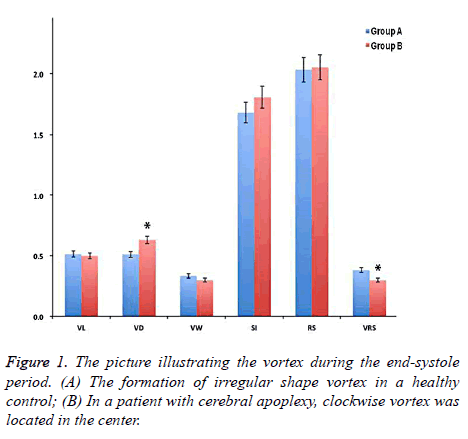
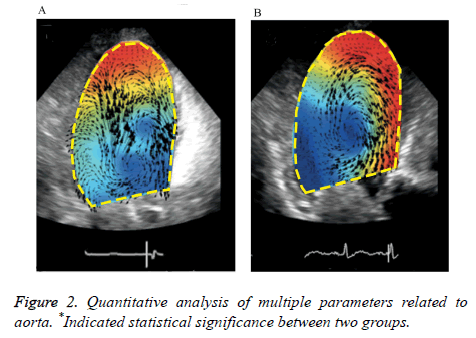
The sequential changes of aorta vortex occurred during cardiac cycle between two groups. In the early systolic phase, it was associated with the left ventricular contraction, axial flow and non-rotational flow. During the middle and late stages, spiral axial flow was noted, which formed a clockwise vortex in the central part of the aorta and the range of vortex extended to peripheral aorta in the control group. A clockwise vortex was also formed in aorta center in the cerebral apoplexy group, it was located in the center but it did not extend to the aorta margins. At the end of the systolic period, the clockwise vortex that was formed in the middle and late stages of contraction collided with new formation of counter clockwise vortex. The irregular shaped vortex was observed in the periphery of the aorta. On the contrary, there was no change of the clockwise vortex in the cerebral apoplexy group and no significant peripheral vortex was generated (Figure 1). For the ultrasound analysis, the interobserver variability coefficient was ranged from -0.11/+0.13, and the intraobserver variability coefficient was between -0.15 and +0.14, suggesting a good inter/ intraobserver correlation in ultrasound examination.
Quantitative analysis of aortic vortex
There was no significant difference in the vortex width which was associated with vortex form, vortex length, vortex sphericity index and other parameters, whereas Vortex Depth (VD) in the control group was significantly deeper than that in the cerebral apoplexy group (0.612 ± 0.171 vs. 0.582 ± 0.122, P<0.05). Relative Strength (RS) represented the pulsation of the whole aorta, there was no significant difference between two groups; The RS of vortex in the cerebral apoplexy group was higher compared with that in the control group (0.362 ± 0.091 vs. 0.381 ± 0.161, P<0.05) (Figure 2).
Discussion
In this study, the characteristics of aortic vortex between the healthy controls and the patients with cerebral apoplexy were statistically compared. The cross-section images of the vortex were utilized to quantify the relative characteristics of vortex, and significant differences were observed among different fluid models between the healthy subjects and patients with cerebral apoplexy.
The flow pattern and hemodynamics of the aorta in thoracic aorta have been intensively investigated. In previous studies, aortic flow was assessed by in vitro application of resin bead or Doppler color echocardiography. Because the fluid model was relatively complex, it could not be applied to evaluate the aortic flow of the human body [12,13]. Previous investigation demonstrated that elevated heart rate could negatively influence cardiovascular risk in the general population. It can induce and promote the atherosclerotic process by means of several mechanisms involving endothelial shear stress and biochemical activities. Furthermore, elevated heart rate can directly increase heart ischemic conditions because of its skill in unbalancing demand/supply of oxygen and decreasing the diastolic period [14]. The computational fluid dynamics assessed by three dimensional numerical imaging has been gradually applied to analyse hemodynamic characteristics of the aorta in vivo. However, several limitations have to be acknowledged. For instance, it was limited by the assumptions of geometric model, viscosity, expansion and flow conditions and other factors [15,16]. Applying and comparing TEE and PIV had multiple advantages for evaluating the characteristics of aortic vortex. It could achieve real-time visualization of aortic flow at a higher resolution after using contrast medium. Meantime, the changes of aortic vortex also possessed a very high temporal resolution in each cardiac cycle at 60-80 frames/ sec.
At present, aortic flow has been widely studied besides simple pulsation, axial position, secondary helix and vortex. The shear stress generated by helical and reverse flow of vortex adjacent to the aortic wall might be one of the most important reasons leading to aortosclerosis [13,14,17,18]. By TEE and color Doppler ultrasound, aortic vortex formed on the cross-section imaging of the descending thoracic aorta. Clockwise or counter-clockwise rotation of the vortex was observed in a majority of patients during paradoxical expansion and relaxation period. Due to the technical limitations, merely the existence of aorta vortex was confirmed. In addition, the indication of TEE was diversified in enrolled patients who were complicated with vascular heart disease or valve prosthesis or alternative complications, but the clinical data regarding healthy controls are lacking. Therefore, the overall quality of this investigation is limited. In this study, the sequential changes of aortic vortex throughout the cardiac cycle were monitored and statistically compared between the healthy controls and cerebral apoplexy patients. In the control group, the clockwise vortex was found during the late stage of contraction contrasted with the counter-clockwise vortex which formed in the early diastole stage. A large quantity of peripheral vortexes was seen at the late stage of contraction, which probably resulted from the shear stress and preventing blood flow into the periphery of the aorta, suggesting a potential role in anti-atherosclerosis. However, the clockwise vortex in the patients with cerebral apoplexy which formed at the late stage of contraction did not coincide with counterclockwise vortex in the early diastole stage, indicating it could endure to the late diastolic stage. Unidirectional great vortex was formed in aortic center without interference of reverse vortex. The RS of vortex in patients with cerebral apoplexy was also higher than that in healthy controls. Blood flow was relatively stagnated around aorta, which increased the potential risk of atherosclerosis.
The lack of counter-clockwise vortex in patients with cerebral apoplexy is probably associated with the decreased divergence rate of aorta. The aortic wall was composed of elastic fibers, which expanded during the contraction. It functions like a blood flow storage device, which pumps and restores the blood flow during the period of relaxation [19-21]. So the form of counter-clockwise vortex during the period of relaxation could be found in the healthy subjects. The pushing effect of patients with cerebral apoplexy in relaxation period was weakened or disappeared, so there was no formation of counter-clockwise vortex. The central position of aorta only reserved in fast flow model, the peripheral blood flow was relatively slow or stagnated.
Shear stress in blood vessels played an important role in atherosclerosis and plaque formation. The studies showed that the weakening of the transverse shear force of the arterial wall was related to atherosclerosis [22-25]. In normal control group, we could find that there was a dynamic and multi direction vortex in the whole cross section area, which included peripheral area of aorta. In the patients with cerebral apoplexy, the vortex in a single direction was only observed in the central region of the aorta. The loss of vortexes in the surrounding area might relate to transverse low shear stress, so that increased the risk of atherosclerosis.
Another important factor that related to atherosclerosis was the transport of Low Density Lipoprotein (LDL). Peripheral blood stagnation and the lack of the formation of the reverse vortex of patients with cerebral apoplexy could increase the time and possibility of blood contacting vascular endothelium, so that it would be easier for low density lipoprotein to be delivered to artery endothelial and further promoted the incidence of atherosclerosis.
In conclusion, application of trans-esophageal ultrasonography could effectively assess the form and varying parameters related to the aortic vortex. The parameters related to the aortic vortex significantly differ between the healthy controls and patients with cerebral apoplexy.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgement
None
References
- Tunick PA, Nayar AC, Goodkin GM, Mirchandani S, Francescone S, Rosenzweig BP, Freedberg RS, Katz ES, Applebaum RM, Kronzon I, NYU Atheroma Group. Effect of treatment on the incidence of stroke and other emboli in 519 patients with severe thoracic aortic plaque. Am J Cardiol 2002; 90: 1320-1325.
- Gimbrone MA, García-Cardena G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc Pathol 2013; 22: 9-15.
- Caro CG. Discovery of the role of wall shear in atherosclerosis. Arterioscler Thromb Vasc Biol 2009; 29: 158-161.
- Heo KS, Fujiwara K, Abe J. Shear stress and atherosclerosis. Mol Cells 2014; 37: 435-440.
- Cecchi E, Giglioli C, Valente S, Lazzeri C, Gensini GF, Abbate R, Mannini L. Role of hemodynamic shear stress in cardiovascular disease. Atherosclerosis 2011; 214: 249-256.
- Vincent PE, Plata AM, Hunt AA, Weinberg PD, Sherwin SJ. Blood flow in the rabbit aortic arch and descending thoracic aorta. J R Soc Interface 2011; 8: 1708-1719.
- Frydrychowicz A, Berger A, Munoz DRA, Russe MF, Bock J, Harloff A, Markl M. Interdependencies of aortic arch secondary flow patterns, geometry, and age analysed by 4-dimensional phase contrast magnetic resonance imaging at 3 Tesla. Eur Radiol 2012; 22: 1122-1130.
- Chen WD, Chen M, Miao AY. Application of flow vector flow mapping technique in the analysis of left ventricular vortex strength (VI) in patients with left ventricular failure. Harbin, Heilongjiang, China 2012; 2012.
- Zhang J, Wang J, Tong X. Study on the characteristics of left ventricle in patients with dilated cardiomyopathy. J PLA Med Coll 2013; 1: 24-27.
- Markl M, Kilner PJ, Ebbers T. Comprehensive 4D velocity mapping of the heart and great vessels by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2011; 13: 7.
- Burk J, Blanke P, Stankovic Z, Barker A, Russe M, Geiger J, Frydrychowicz A, Langer M, Markl M. Evaluation of 3D blood flow patterns and wall shear stress in the normal and dilated thoracic aorta using flow-sensitive 4D CMR. J Cardiovasc Magn Reson 2012; 14: 84.
- Bogren HG, Buonocore MH, Valente RJ. Four-dimensional magnetic resonance velocity mapping of blood flow patterns in the aorta in patients with atherosclerotic coronary artery disease compared to age-matched normal subjects. J Magn Reson Imaging 2004; 19: 417-427.
- Sengupta PP, Pedrizetti G, Narula J. Multiplanar visualization of blood flow using echocardiographic particle imaging velocimetry. JACC Cardiovasc Imaging 2012; 5: 566-569.
- Scicchitano P, Cortese F, Ricci G, Carbonara S, Moncelli M. Ivabradine, coronary artery disease, and heart failure: beyond rhythm control. Drug Des Devel Ther 2014; 8: 689-700.
- Frydrychowicz A, Markl M, Hirtler D, Harloff A, Schlensak C, Geiger J, Stiller B, Arnold R. Aortic hemodynamics in patients with and without repair of aortic coarctation: in vivo analysis by 4D flow-sensitive magnetic resonance imaging. Invest Radiol 2011; 46: 317-325.
- Sengupta PP, Pedrizzetti G, Kilner PJ, Kheradvar A, Ebbers T. Emerging trends in CV flow visualization. JACC Cardiovasc Imaging 2012; 5: 305-316.
- Chou P, Yao BN, Wang ZH, Han SQ, Li JX, Xu P, Ji YY. Evaluation of left ventricular function in patients with different parts of right ventricular pacing by eddy current vector imaging. Chin J Clinic 2014; 23: 4191-4195.
- Kheradvar A, Houle H, Pedrizzetti G, Tonti G, Belcik T, Ashraf M, Lindner JR, Gharib M, Sahn D. Echocardiographic particle image velocimetry: a novel technique for quantification of left ventricular blood vorticity pattern. J Am Soc Echocardiogr 2010; 23: 86-94.
- Hong GR, Pedrizzetti G, Tonti G, Li P, Wei Z, Kim JK, Baweja A, Liu S, Chung N, Houle H, Narula J, Vannan MA. Characterization and quantification of vortex flow in the human left ventricle by contrast echocardiography using vector particle image velocimetry. JACC Cardiovasc Imaging 2008; 1: 705-717.
- Svedlund S, Wetterholm R, Volkmann R, Caidahl K. Retrograde blood flow in the aortic arch determined by transesophageal Doppler ultrasound. Cerebrovasc Dis 2009; 27: 22-28.
- Peiffer V, Sherwin SJ, Weinberg PD. Computation in the rabbit aorta of a new metric-the transverse wall shear stress-to quantify the multidirectional character of disturbed blood flow. J Biomech 2013; 46: 2651-2658.
- Wada S, Karino T. Theoretical prediction of low-density lipoproteins concentration at the luminal surface of an artery with a multiple bend. Ann Biomed Eng 2002; 30: 778-791.
- Warboys CM, Amini N, de Luca A, Evans PC. The role of blood flow in determining the sites of atherosclerotic plaques. F1000 Med Rep 2011; 3: 5.
- Zhang Y, Ding YC, Wang Q. Quantitative assessment of left ventricular vortex characteristics in normal adults by flow vector imaging. J Kunming Med Univ 2015; 10: 36-40.
- Ma XJ, Wang B, Xia J. Preliminary study of flow vector imaging in the right atrium and right ventricle in normal subjects. Chin J Clinic 2013; 17: 7743-7747.