Research Article - Biomedical Research (2018) Volume 29, Issue 4
Colitis reducing effects of Lactobacillus plantarum YS-4 in dextran sulfate sodium-induced C57BL/6J mice
Guijie Li1,2,3,4#, Xingyao Long5#, Yanni Pan1,2, Xiao Meng6 and Xin Zhao1,2,3*
1Chongqing Collaborative Innovation Center for Functional Food, Chongqing University of Education, Chongqing, PR China
2Chongqing Engineering Research Center of Functional Food, Chongqing University of Education, Chongqing, PR China
3Chongqing Engineering Laboratory for Research and Development of Functional Food, Chongqing University of Education, Chongqing, PR China
4College of Biological and Chemical Engineering, Chongqing University of Education, Chongqing, PR China
5College of Food Science, Southwest University, Chongqing, PR China
6School of Public Health, Chengdu University of TCM, Chengdu, Sichuan, PR China
#These authors have contributed equally to this work
- *Corresponding Author:
- Xin Zhao
Chongqing Collaborative Innovation Center for Functional Food
Chongqing University of Education, PR China
Accepted date: November 20, 2017
DOI: 10.4066/biomedicalresearch.29-17-3436
Visit for more related articles at Biomedical ResearchAbstract
This study observed the inhibitory effect of Lactobacillus plantarum YS-4 (LP-YS4) on the colitis through mice colitis which was induced by DDS (Dextran Sulfate Sodium). By observing the colon shape of mice, the test kit and quantitative PCR (polymerase chain reaction) technique were used to detect the mice, and the effect of LP-YS4 was compared with the positive control of drug sulfasalazine. The results showed that LP-YS4 can increase the colon length of colitis mice and improve the ratio of colon weight/ colon length. The tests on serum of mice showed that LP-YS4 could reduce Endothelin (ET), Substance P (SP), Interleukin-10 (IL-10) levels and increase Somatostatin (SS), Vasoactive Intestinal Peptide (VIP), Interleukin-2 (IL-2) levels in serum of colitis mice. The tests on colon tissue in mice showed that LP-YS4 could improve Glutathione (GSH), Superoxide Dismutase (SOD) activities and decrease Myeloperoxidase (MPO), Malonaldehyde (MDA) activities in the colon of colitis mice; and LP-YS4 could also increase the expression of neuronal Nitric Oxide Synthase (nNOS), endothelial Nitric Oxide Synthase (eNOS), c-Kit, Stem Cell Factor (SCF) mRNA and decrease the expression of inducible Nitric Oxide Synthase (iNOS), Interleukin-8 (IL-8), Chemokine (C-X-C motif) Receptor 2 (CXCR2) in the colon of colitis mice. It can be seen that LP-YS4 has the effect of colitis suppression, the effect of high concentration LP-YS4 is close to the drug sulfasalazine.
Keywords
Lactobacillus plantarum, Dextran sulfate sodium, Colitis, PCR, Expression
Introduction
Yak yogurt is a kind of natural fermented dairy product which is usually consumed by herdsmen of the minorities in the Qinghai-Tibet Plateau. Some studies have shown that the natural fermented yak yogurt contains variety of nutrients and has many biological activities, which is a kind of high-quality natural food [1]. Yak yogurt is a kind of pure natural fermented food, its quality is affected by raw material and fermentation conditions, specially the difference of microbial fermentation under different environment has a large impact on the quality of yak yogurt. Therefore, there is a certain difference of the antioxidant capacity and immune function of yak milk in different regions [1,2]. The microbial components in Yak yogurt have a large difference from general lactic acid because of the special working environment and fermentation [3]. Yak yogurt contains a large amount of Lactobacillus so that the deep separation and identification can isolate a high-quality Lactobacillus with probiotics potential [4,5].
UC (Ulcerative Colitis) is a kind of inflammatory bowel disease, mainly characterized by abdominal pain, diarrhea and bloody stools. The pathogenesis of UC is not clear, but the incidence rate is high. It’s a common method to study the effect and mechanism of food on UC by animal model [6]. In which the DDS model can form a similar expression of clinical manifestations of UC is the most ideal model of ulcerative colitis [7]. Long-term exposure to oxidative stress will lead to intestinal damage, but part of lactic acid bacteria in the intestine can play an antioxidant role, remove free radicals and ease UC [8]. Intestinal mucosa permeability will change as the intestinal tract gradually becomes inflammatory, and the mucous membrane permeability will be greatly improved when inflammation is increased [9,10]. Study has shown that some lactic acid bacteria can alleviate DSS-induced colitis by improving the permeability of intestinal mucosa [11]. Clinical studies have also reported that Lactobacillus can significantly assist in the treatment of UC drug therapy [12]. Lactobacillus can relieve UC in many ways, but the study of its mechanism is lack in depth. At the same time, there is no research on the probiotic potential of lactic acid bacteria.
In this study, the object is Lactobacillus plantarum YS-4 (LPYS4) which was isolated and identified from the natural fermentation of the Tibetan autonomous prefecture of Yushu in Qinghai province in China to observe the inhibitory effect of LP-YS4 on DSS-induced colitis. Using molecular biology methods, especially to observe colon related changes in gene expression, detect the LP-YS4 influence on colitis of experimental animals and verify the LP-YS4 inhibition effect on DSS induced colitis to accumulate theory basis for further use of LP-YS4.
Materials and Methods
Microorganism strains
Lactobacillus plantarum YS4 (preservation number: M2016747) was preserved in China Center for Type Culture Collection (CCTCC, Wuhan, China), which was identified from yak yoghurt produced in the Yushu (Qinghai Province, China).
Experimental animal
Seven weeks old male C57BL/6J mice were purchased from the experimental animal center of Chongqing Medical University (license number: SYXK (Chongqing) 2012-0001). Mice were reared at room temperature environment at 25 ± 2°C and the relative humidity in the rearing environment at 50 ± 5% while maintaining every 12 h regulation light/dark.
Animal experiment
After a week of feeding, selecting 50 of C57BL/6J mice in weight of 25 ± 2 g and dividing them into 5 groups, which were named normal group, model group, LP-YS4-L, LP-YS4- H group and SASP (sulfasalazine, Sigma-Aldrich Corp. St Louis, MO, USA) group (positive control group). During the 5 w experimental period, the normal group mice were given free drinking water and diet; the model group were given 2% and 4% (w/v) DSS (Sigma-Aldrich) water solution respectively in w 3 and w 5, and then given the free drinking water and diet in the remaining time; LP-YS4-L and LP-YS4-H groups mice were given 0.2 ml 1 × 108 CFU/ml and 1 × 109 CFU/ml LPYS4 per day except for free drinking water and diet. SASP group mice were given SASP with a concentration of 20 mg/kg b.w. [13] per day in addition to free drinking water and diet. The weight and length of the colon were measured, blood and colon tissues were taken for further use when the mice were executed by broken neck way after 5 w experiment.
Determination of serum ET-1, SS, SP and VIP levels in mice
The levels of ET-1, SS, SP and VIP in serum were determined by kits (Beijing Puerweiye biological science and Technology Co., Ltd., Beijing, China) ways after 15 min 4500 rpm centrifugation [14].
Determination of serum cytokine IL-2 and IL-10 levels in mice
The cytokine levels of IL-2 and IL-10 in serum were determined by kits (Thermo Fisher Scientific, Waltham, MA, USA) ways after 15 min 4500 rpm centrifugation [14].
Determination of activities of MPO, GSH, MDA and SOD in colon tissue
The colon tissue and normal saline was mixed by 1:9, and then colon tissue was homogenized by ultrasonic pulverization. The activities of MPO, GSH, MDA and SOD in colon tissue were determined by kit method [14].
Determination of mRNA expression in colon tissue by qPCR assay
The colon tissue of the mouse was smashed and then RNAzol was used to extract the total RNA of the colon tissue, and the total RNA concentration extracted was diluted to 1 μg/μL. Taking 5 μL diluted total RNA extract, obtaining cDNA template by reverse transcription kit method. A 2 μL cDNA template was mixed with 10 μL of SYBR Green PCR Master Mix and 1 μL each of upstream and downstream primers (Table 1). At the reaction condition of 95°C, conducting 40 cycles after 60 s: 95°C, 15 s; 55°C, 30 s; 72°C, 35 s and 95°C, 30 s; testing at 55°C, 35 s, GAPDH was taken as reference, the relative expression of gene was counted using the formula 2- ΔΔCt=ΔCt (gene detection)-ΔCt (GAPDH) [15].
| Gene | Primer sequence |
|---|---|
| nNOS | Forward primer: 5'-ATGTCCTCAAAGCCATCCAG-3' |
| Reverse primer: 5'-ACTCAGATCTAAGGCGGTTG-3' | |
| eNOS | Forward primer: 5'-TGTCTGCGGCGATGTCACT-3' |
| Reverse primer: 5'-CATGCCGCCCTCTGTTG-3' | |
| iNOS | Forward primer: 5'-CAGCTGGGCTGTACAAACCTT-3' |
| Reverse primer: 5'-CATTGGAAGTGAAGCGTTTGG-3' | |
| c-Kit | Forward primer: 5'-CATAGCCCAGGTAAAGCACAAT-3' |
| Reverse primer: 5'-GAACACTCCAGAATCGTCAACTC-3' | |
| SCF | Forward primer: 5'-TCAGGGACTACGCTGCGAAAG-3' |
| Reverse primer: 5'-AAGAGCTGGCAGACCGACTCA-3' | |
| IL-8 | Forward primer: 5'-CTAGGCATCTTCGTCCGTCC-3' |
| Reverse primer: 5'-TTGGGCCAACAGTAGCCTTC-3' | |
| CXCR2 | Forward primer: 5'-TCTGCTCACAAACAGCGTCGTA-3' |
| Reverse primer: 5'-GAGTGGCATGGGACAGCATC-3' | |
| GAPDH | Forward primer: 5'-TGCACCACCAACTGCTTAG-3' |
| Reverse primer: 5'-GATGCAGGGATGATGTTC-3' |
Table 1. Sequences of reverse transcription polymerase chain reaction primers used in this study.
Statistical analysis
The mean ± SD was represented the data. Differences between the mean values for individual groups were assessed by oneway Analysis of Variance (ANOVA) with the Student- Neumann-Keuls post-hoc test by SPSS software 19.0 (IBM Software, Armonk, NY). p<0.05 was considered a statistically significant difference.
Results
Colon length and colon weight
As shown in Table 2, the normal group mice had the highest colon length and colon weight/colon length; Lactobacillus plantarum YS-4 treated mice had the higher colon length and colon weight/colon length than model group mice, and LP-YSH treated mice showed those data only lower than normal group and SASP group mice.
| Group | Colon length (cm) | Colon weight/colon length(mg/cm) |
|---|---|---|
| Normal | 9.5 ± 0.3a | 43.4 ± 4.2a |
| Model | 3.7 ± 0.4e | 14.8 ± 2.1e |
| LP-YS4-L | 5.2 ± 0.4d | 26.6 ± 2.3d |
| LP-YS4-H | 7.1 ± 0.4c | 32.8 ± 2.7c |
| SASP | 8.2 ± 0.3b | 37.9 ± 2.2b |
Table 2. Colon length and colon weight/colon length of each group mice.
Serum ET-1, SS, SP and VIP levels in mice
As shown in Table 3, the mice in model group showed the highest ET-1, SP and lowest SS, VIP levels, after treated with LP-YS4, the ET-1, SP levels were decreased and SS, VIP levels were increased in colitis mice. Meanwhile ET-1, SP levels of the high concentration of LP-YS4 (LP-YS4-H) treated mice were lower than low concentration of LP-YS4 (LP-YS4- L) treated mice, but SS, VIP levels were higher than those in LP-YS4-L treated mice.
| Group | ET (pg/mL) | SS (pg/ml) | SP (pg/ml) | VIP (pg/mL) (nmol/mg) |
|---|---|---|---|---|
| Normal | 7.03 ± 0.26e | 55.87 ± 3.21a | 40.01 ± 1.27e | 64.28 ± 3.70a |
| Model | 19.08 ± 0.51a | 22.71 ± 3.02e | 70.81 ± 3.76a | 26.18 ± 2.36e |
| LP-YS4-L | 15.09 ± 0.43b | 30.87 ± 2.25d | 58.29 ± 2.19b | 37.10 ± 2.66d |
| LP-YS4-H | 10.77 ± 0.31c | 40.36 ± 3.12c | 50.33 ± 2.24c | 50.37 ± 2.43c |
| SASP | 8.87 ± 0.25d | 46.21 ± 2.18b | 45.28 ± 2.03d | 57.12 ± 2.19b |
Table 3. ET, SS, SP and VIP serum levels of each group mice.
Serum IL-2 and IL-10 cytokine levels in mice
As shown in Table 4, the mice in normal group had the highest IL-2 level and lowest IL-10 level; after inducing colitis by DSS, the IL-2 level was reduced but IL-10 was raised in mice. LP-YS4-H treatment showed the strong IL-2 level increasing effect and IL-10 level decreasing effect in colitis mice, LPYS4- H made the mice had the higher IL-2 level and lower IL-10 level than those in LP-YS4-L treated mice and model group mice.
| Group | IL-2 (pg/ml) | IL-10 (pg/ml) |
|---|---|---|
| Normal | 266.90 ± 34.28a | 117.837 ± 16.38e |
| Model | 66.31 ± 20.52e | 882.39 ± 30.56a |
| LP-YS4-L | 108.92 ± 24.79d | 623.17 ± 22.53b |
| LP-YS4-H | 182.19 ± 26.32c | 343.29 ± 24.33c |
| SASP | 212.38 ± 22.61b | 226.18 ± 31.28d |
Table 4. IL-2 and IL-10 serum levels of each group mice.
Colon tissue MPO, GSH, MDA and SOD activities in mice
As shown in Table 5, the mice in normal group showed the highest GSH, SOD activities and lowest MPO, MDA activities, but the mice in model group showed the lowest GSH, SOD activities and highest MPO, MDA activities. The GSH, SOD activities of mice in LP-YS4-H group were higher than those of mice in LP-YS4-L group, but lower than those of mice in SASP group; meanwhile the MPO, MDA activities of mice in LP-YS4-H group were lower than those of mice in LP-YS4-L group, but higher than those of mice in SASP group.
| Group | MPO (mU/mg) | GSH (µmol/mg) | MDA (nmol/mg) | SOD (U/mg) |
|---|---|---|---|---|
| Normal | 6.15 ± 0.10e | 8.25 ± 0.24a | 0.32 ± 0.04e | 94.12 ± 4.37a |
| Model | 35.16 ± 3.56a | 2.36 ± 0.29e | 1.71 ± 0.24a | 22.38 ± 2.49e |
| LP-YS4-L | 22.17 ± 2.08b | 3.66 ± 0.25d | 1.22 ± 0.19b | 42.09 ± 3.02d |
| LP-YS4-H | 15.26 ± 2.03c | 5.76 ± 0.28c | 0.81 ± 0.12c | 66.18 ± 3.12c |
| SASP | 10.23 ± 1.45d | 6.86 ± 0.21b | 0.57 ± 0.14d | 78.38 ± 3.24b |
Table 5. MPO, GSH, MDA and SOD colon tissue contents of each group mice.
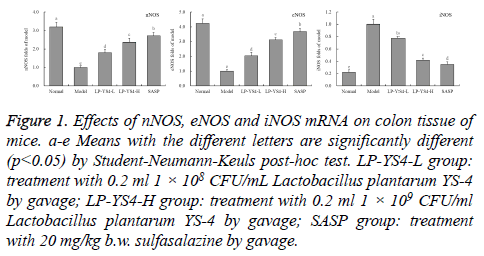
nNOS, eNOS and iNOS mRNA expression of colon tissue in mice
As shown in Figure 1, the mice in normal group showed the strongest nNOS (3.19 folds of model group), eNOS (4.22 folds of model group) mRNA expressions but weakest iNOS (0.22 folds of model group) expression. The mice in LP-YS4-H group also showed stronger nNOS (2.36 folds of model group), eNOS (3.11 folds of model group) expressions and weaker iNOS (0.42 folds of model group) expression than those of mice in LP-YS4-L group (1.79, 2.03 and 0.77 folds of model group) and model group; but the mice in LP-YS4-H group showed weaker nNOS, eNOS expressions and stronger iNOS expression than those of mice in SASP group (2.71, 3.67 and 0.35 folds of model group).
Figure 1: Effects of nNOS, eNOS and iNOS mRNA on colon tissue of mice. a-e Means with the different letters are significantly different (p<0.05) by Student-Neumann-Keuls post-hoc test. LP-YS4-L group: treatment with 0.2 ml 1 × 108 CFU/mL Lactobacillus plantarum YS-4 by gavage; LP-YS4-H group: treatment with 0.2 ml 1 × 109 CFU/ml Lactobacillus plantarum YS-4 by gavage; SASP group: treatment with 20 mg/kg b.w. sulfasalazine by gavage.
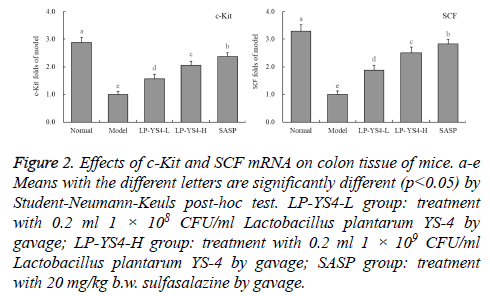
c-Kit and SCF mRNA expression of colon tissue in mice
As shown in Figure 2, the mice in model group showed the weakest c-Kit and SCF mRNA expressions, but the mice in normal group had the strongest c-Kit (2.89 folds of model group) and SCF (3.29 folds of model group) expressions. The mice in SASP group also showed the strong c-Kit (2.37 folds of model group) and SCF (2.83 folds of model group) expressions, and these expressions were stronger than those of mice in LP-YS4-H (2.06 and 2.51 folds of model group) and LP-YS4-L (1.56 and 1.88 folds of model group) group.
Figure 2: Effects of c-Kit and SCF mRNA on colon tissue of mice. a-e Means with the different letters are significantly different (p<0.05) by Student-Neumann-Keuls post-hoc test. LP-YS4-L group: treatment with 0.2 ml 1 × 108 CFU/ml Lactobacillus plantarum YS-4 by gavage; LP-YS4-H group: treatment with 0.2 ml 1 × 109 CFU/ml Lactobacillus plantarum YS-4 by gavage; SASP group: treatment with 20 mg/kg b.w. sulfasalazine by gavage.
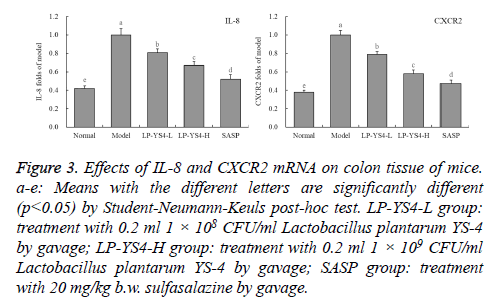
IL-8 and CXCR2 mRNA expression of colon tissue in mice
As shown in Figure 3, the DSS induced colitis mice (model group) showed the strongest IL-8 and CXCR2 mRNA expressions, but the normal mice (normal group) showed the weakest IL-8 (0.42 folds of model group) and CXCR2 (0.38 folds of model group) expressions. After treatment with LPYS4, the IL-8 and CXCR2 expressions in colitis mice were reduced compared to the mice in model group, LP-YS4-H treated mice showed weaker IL-8 (0.67 folds of model group) and CXCR2 (0.58 folds of model group) expressions than LPYS4- L (0.81 and 0.79 folds of model group) treated mice. And the drug of SASP showed the stronger IL-8 and CXCR2 expressions reducing effects than LP-YS4-H, the IL-8 (0.52 folds of model group) and CXCR2 (0.47 folds of model group) expressions of mice in SASP group were only stronger than those of mice in normal group.
Figure 3: Effects of IL-8 and CXCR2 mRNA on colon tissue of mice. a-e: Means with the different letters are significantly different (p<0.05) by Student-Neumann-Keuls post-hoc test. LP-YS4-L group: treatment with 0.2 ml 1 × 108 CFU/ml Lactobacillus plantarum YS-4 by gavage; LP-YS4-H group: treatment with 0.2 ml 1 × 109 CFU/ml Lactobacillus plantarum YS-4 by gavage; SASP group: treatment with 20 mg/kg b.w. sulfasalazine by gavage.
Discussion
DSS-induced mice have a different colon weight and length compared with the normal mice. Therefore, the ratio of colon weight/colon length was taken as an important criterion for the degree of experimental ulcerative colitis, and ulcerative colitis mice colon length was lower than that of normal mice, and the ratio of colon weight/colon length was lower than normal mice [16].
Endothelin is a long-lasting and the most effective vasoactive skin substance [17]. Study has shown that the vasoconstriction of ET will cause the erosion of the colonic mucosa, which will cause ulcers in serious cases and play a key role in the process of pathogenesis of ulcerative colitis [18]. SS is an important gastrointestinal hormone, which can suppress the secretion of gastric intestinal juice. The decrease of SS will aggravate the secretion of gastric intestinal juice and aggravate gastrointestinal inflammation [19]. Substance P is a kind of never skin substance with the usage of regulation. Through the regulation of substance P on the nervous system and immune system, it may have certain regulation effect on colitis. The accumulation of substance P can aggravate the degree of colitis [18]. At the same time, a study has shown that P can induce colitis, the antagonistic of substance P can relieve the inflammation of enteritis in animals [20]. The results show that the level of VIP is closely related to the degree of colitis, and the decrease of VIP level will directly lead to the disturbance of immune regulation and aggravation of inflammation in ulcerative colitis [19]. At the same time, VIP can inhibit the transcription of iNOS in the body, and avoid the damage of the intestinal mucosa caused by the transformation of the iNOS from the colon into excessive NO [21]. It can be seen that LPYS4 can inhibit ulcerative colitis by reducing ET, SP level and increasing SS, VIP level, and its role is close to drug sulfasalazine.
IL-2 is a cytokine secreted by Th2, which is directly related to UC; Th2 cell mediated immune response affects UC, and IL-2 plays a role in inhibiting inflammation by affecting Th2 cells, and reducing the degree of UC [22-24]. IL-10 is a cytokine secreted by Treg cells, which has an immunosuppressive effect. In the process of UC inflammation, it acts as a proinflammatory role and exacerbates UC. LP-YS4 can enhance IL-2 levels and reduce IL-10 levels, which can inhabit the inflammation and play a role in reducing the UC process [24].
When UC leads to inflammation of the colon, the concentration of neutrophils in the inflammatory tissues begins to decline, and more neutrophils flow into the tissue, which is manifested as a significant increase in MPO activity [25]. UC increases a large number of free radicals in the colon, including ROS and RNS. A large number of these free radicals make colon tissue damage and toxic reaction, with the increase of free radicals, the degree of inflammation of colitis is also increasing [26,27]. Clinical studies have also shown that UC can lead to dysfunction of the colon in patients with oxidative stress, and SOD decomposition of free radical activity decreased [28]. The results of this study also confirmed that UC led to decreased activity of SOD and GSH, while MPO and MDA activity enhanced, LP-YS4 could significantly inhibit the oxidative stress response of UC to the colon and inhibit colitis.
A study has shown that eNOS can control the production of the right amount of NO, so that to keep the colon tissue in normal condition and play an important control effect on the colon injury caused by colitis. INOS can turn out a large number of NO, and excess NO has side effects on the lesions of colonic tissue [29]. nNOS can also control the concentration of NO in the tissue, and keep the tissue from being infringed by excessive NO. At the same time, nNOS can control the excess expression of iNOS and inhibit inflammation [29,30]. In this study, LP-YS4-H mice were up-regulated by the expression of nNOS and eNOS in colon and down regulated the expression of iNOS, which inhibited the colitis.
UC leads to the dysfunction of the colon and the decrease of colon power. UC leads to a decrease in the number of normal Cajal mesenchymal cells, which resulting in a decrease of colonic power and it has a direct association with the UC process [31]. c-Kit and SCF play an important role in maintaining the number of interstitial cells of Cajal. After the SCF / Kit signal pathway is impaired, the number of interstitial cells of Cajal is not only decreased, but also the proliferation of Cajal interstitial cells will be affected, and the symptoms of UC will be exacerbated [32]. The experimental results showed that LP-YS4 could inhibit DSS-induced colitis by increasing the expression of c-kit and SCF in colon tissues.
IL-8 is a kind of neutrophil chemotaxis and activation factor, which can mediate the inflammatory response induced by various pathways, including the ability to mediate the inflammation of UC [33]. CINC-1 is one of the family of IL-8 chemotactic agents for neutrophils, CXCR2 is CINC-1 receptor, CXCR2 can play a role of medium to regulate interactions between organs, reduce the content of CXCR2 in the organization can help to alleviate the colon injury caused by UC [34,35]. LP-YS4 can significantly reduce the expression of IL-8 and CXCR2 mRNA in the colon of colitis mice, thus inhibiting colitis.
Conclusions
In this study, DSS-induced colitis model of mice was used to study the inhibition effect of lactobacillus of LP-YS4 which was isolated from natural fermented yak milk. Through the observation of the serum and colon tissue in mice, the experimental results showed that the model group compared with colitis mice, LP-YS4 can inhibit colon length shortening and colon weight caused by colitis/ratio decrease in the length of the colon. It was found that LP-YS4 can reduce ET, SP level and increase SS and VIP level in serum of mice with colitis through the test kit. At the same time, LP-YS4 can also improve the levels of IL-2 cytokines and reduce IL-10 levels in serum. After analyzing the colonic tissue, it can be seen that LP-YS4 can enhance GSH, SOD activity and decrease MPO and MDA activity in colon tissues of colitis mice, and inhibit the oxidative stress response caused by DSS. The further qPCR results showed that LP-YS4 can increase the expression of nNOS, eNOS, c-Kit, SCF mRNA and decrease the expression of iNOS, IL-8 and CXCR2 in the colon of colitis mice. It can be seen from the results, LP-YS4 can inhibit DSS-induced colitis, the concentration and effect are positively correlated, and the role of high concentration LP-YS4 is close to the treatment of drug sulfasalazine. LP-YS4 is a high-quality Lactobacillus with colitis inhibiting effect, which should be further developed and used.
Acknowledgements
The present research was supported by Chongqing Research Program of Basic Research and Frontier Technology (cstc2016jcyjA0339), Chongqing Engineering Research Center for Functional Food (grant no., cstc2015yfpt_gcjsyjzx0027), Research Project of Chongqing University of Education (KY2015TBZC), and the Program for Innovation and Team Building at the Chongqing Institute of Higher Education (grant no. CXTDX201601040), China.
Conflict of Interest
There is no conflict of interest.
References
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696-1705.
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012; 55: 1577-1596.
- Ahmad SR, Swann J. Exenatide and rare adverse events. N Engl J Med 2008; 358: 1970-1971.
- Ayoub WA, Kumar AA, Naguib HS, Taylor HC. Exenatide-induced acute pancreatitis. Endocr Pract 2010; 16: 80-83.
- Denker PS, Dimarco PE. Exenatide (exendin-4)-induced pancreatitis: a case report. Diabetes Care 2006; 29: 471.
- Tripathy NR, Basha S, Jain R, Shetty S, Ramachandran A. Exenatide and acute pancreatitis. J Assoc Physicians India 2008; 56: 987-988.
- Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011; 141: 150-156.
- Singh S, Chang HY, Richards TM, Weiner JP, Clark JM, Segal JB. Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med 2013; 173: 534-539.
- Azoulay L, Filion KB, Platt RW, Dahl M, Dormuth CR, Clemens KK, Durand M, Hu N, Juurlink DN, Paterson JM, Targownik LE, Turin TC, Ernst P, Suissa S, Dormuth CR, Hemmelgarn BR, Teare GF, Caetano P, Chateau D, Henry DA, Paterson JM, LeLorier J, Levy AR, Ernst P, Platt RW, Sketris IS. Association between incretin-based drugs and the risk of acute pancreatitis. JAMA Intern Med 2016; 176: 1464-1473.
- Azoulay L, Filion KB, Platt RW, Dahl M, Dormuth CR, Clemens KK, Durand M, Juurlink DN, Targownik LE, Turin TC, Paterson JM, Ernst P. Incretin based drugs and the risk of pancreatic cancer: international multicentre cohort study. BMJ 2016; 352: 581.
- Knapen LM, de Jong RG, Driessen JH, Keulemans YC, van Erp NP, De Bruin ML, Leufkens HG, Croes S, de Vries F. Use of incretin agents and risk of acute and chronic pancreatitis: A population-based cohort study. Diabetes Obes Metab 2017; 19: 401-411.
- Storgaard H, Cold F, Gluud LL, Vilsboll T, Knop FK. Glucagon-like peptide-1 receptor agonists and risk of acute pancreatitis in patients with type 2 diabetes. Diabetes Obes Metab 2017; 19: 906-908.
- Butler AE, Galasso R, Matveyenko A, Rizza RA, Dry S, Butler PC. Pancreatic duct replication is increased with obesity and type 2 diabetes in humans. Diabetologia 2010; 53: 21-26.
- Martinez J, Johnson CD, Sanchez-Paya J, de Madaria E, Robles-Diaz G, Perez-Mateo M. Obesity is a definitive risk factor of severity and mortality in acute pancreatitis: an updated meta-analysis. Pancreatology 2006; 6: 206-209.
- Noel RA, Braun DK, Patterson RE, Bloomgren GL. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care 2009; 32: 834-838.
- Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 2013; 62: 2595-2604.
- Gier B, Matveyenko AV, Kirakossian D, Dawson D, Dry SM, Butler PC. Chronic GLP-1 receptor activation by exendin-4 induces expansion of pancreatic duct glands in rats and accelerates formation of dysplastic lesions and chronic pancreatitis in the Kras(G12D) mouse model. Diabetes 2012; 61: 1250-1262.
- Koehler JA, Baggio LL, Lamont BJ, Ali S, Drucker DJ. Glucagon-like peptide-1 receptor activation modulates pancreatitis-associated gene expression but does not modify the susceptibility to experimental pancreatitis in mice. Diabetes 2009; 58: 2148-2161.
- Matveyenko AV, Dry S, Cox HI, Moshtaghian A, Gurlo T, Galasso R, Butler AE, Butler PC. Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin. Diabetes 2009; 58: 1604-1615.
- Nachnani JS, Bulchandani DG, Nookala A, Herndon B, Molteni A, Pandya P, Taylor R, Quinn T, Weide L, Alba LM. Biochemical and histological effects of exendin-4 (exenatide) on the rat pancreas. Diabetologia 2010; 53: 153-159.
- Tatarkiewicz K, Belanger P, Gu G, Parkes D, Roy D. No evidence of drug-induced pancreatitis in rats treated with exenatide for 13 weeks. Diabetes Obes Metab 2013; 15: 417-426.
- Tatarkiewicz K, Smith PA, Sablan EJ, Polizzi CJ, Aumann DE, Villescaz C, Hargrove DM, Gedulin BR, Lu MG, Adams L, Whisenant T, Roy D, Parkes DG. Exenatide does not evoke pancreatitis and attenuates chemically induced pancreatitis in normal and diabetic rodents. Am J Physiol Endocrinol Metab 2010; 299: 1076-1086.
- Vrang N, Jelsing J, Simonsen L, Jensen AE, Thorup I, Soeborg H, Knudsen LB. The effects of 13 wk of liraglutide treatment on endocrine and exocrine pancreas in male and female ZDF rats: a quantitative and qualitative analysis revealing no evidence of drug-induced pancreatitis. Am J Physiol Endocrinol Metab 2012; 303: 253-264.
- McMurray F, Cox RD. Mouse models and type 2 diabetes: translational opportunities. Mamm Genome 2011; 22: 390-400.
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487-1495.
- Schmidt J, Lewandrowski K, Fernandez-del Castillo C, Mandavilli U, Compton CC, Warshaw AL, Rattner DW. Histopathologic correlates of serum amylase activity in acute experimental pancreatitis. Dig Dis Sci 1992; 37: 1426-1433.
- Funch D, Gydesen H, Tornoe K, Major-Pedersen A, Chan KA. A prospective, claims-based assessment of the risk of pancreatitis and pancreatic cancer with liraglutide compared to other antidiabetic drugs. Diabetes Obes Metab 2014; 16: 273-275.
- Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Glucagon-like peptide-1 receptor agonists and pancreatitis: a meta-analysis of randomized clinical trials. Diabetes Res Clin Pract 2014; 103: 269-275.
- Monami M, Nreu B, Scatena A, Cresci B, Andreozzi F, Sesti G, Mannucci E. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): Data from randomized controlled trials. Diabetes Obes Metab 2017; 19: 1233-1241.
- Gedulin BR, Nikoulina SE, Smith PA, Gedulin G, Nielsen LL, Baron AD, Parkes DG, Young AA. Exenatide (exendin-4) improves insulin sensitivity and {beta}-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weight. Endocrinology 2005; 146: 2069-2076.
- Rolin B, Larsen MO, Gotfredsen CF, Deacon CF, Carr RD, Wilken M, Knudsen LB. The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab 2002; 283: 745-752.
- Tomas E, Wood JA, Stanojevic V, Habener JF. GLP-1-derived nonapeptide GLP-1(28-36) amide inhibits weight gain and attenuates diabetes and hepatic steatosis in diet-induced obese mice. Regul Pept 2011; 169: 43-48.
- Bauer AS, Keller A, Costello E, Greenhalf W, Bier M, Borries A, Beier M, Neoptolemos J, Buchler M, Werner J, Giese N, Hoheisel JD. Diagnosis of pancreatic ductal adenocarcinoma and chronic pancreatitis by measurement of microRNA abundance in blood and tissue. PLoS One 2012; 7: 34151.
- Endo K, Weng H, Kito N, Fukushima Y, Iwai N. MiR-216a and miR-216b as markers for acute phased pancreatic injury. Biomed Res 2013; 34: 179-188.
- Kong XY, Du YQ, Li L, Liu JQ, Wang GK, Zhu JQ, Man XH, Gong YF, Xiao LN, Zheng YZ, Deng SX, Gu JJ, Li ZS. Plasma miR-216a as a potential marker of pancreatic injury in a rat model of acute pancreatitis. World J Gastroenterol 2010; 16: 4599-4604.
- Mardin WA, Mees ST. MicroRNAs: novel diagnostic and therapeutic tools for pancreatic ductal adenocarcinoma? Ann Surg Oncol 2009; 16: 3183-3189.
- Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, Chevali L. Pathophysiology of acute pancreatitis. Pancreatology 2005; 5: 132-144.
- Faillie JL, Babai S, Crepin S, Bres V, Laroche ML, Le Louet H, Petit P, Montastruc JL, Hillaire-Buys D. Pancreatitis associated with the use of GLP-1 analogs and DPP-4 inhibitors: a case/non-case study from the French pharmacovigilance database. Acta Diabetol 2014; 51: 491-497.