Case Report - Journal of Neurology and Neurorehabilitation Research (2017) Volume 2, Issue 1
Cognitive enhancement exciting discovery using trans-lingual neuro-stimulation
- *Corresponding Author:
- Dafna Paltin
University of Wisconsin, Madison USA
Tel: 6108043452
Fax: 6108043452
E-mail: dpaltin@gmail.com
Accepted date: April 24, 2017
Citation: Paltin D, Tyler M, Danilov Y. Cognitive enhancement exciting discovery using trans-lingual neuro-stimulation. J Neurol Neurorehabil Res.2017;2(1):39-45.
DOI: 10.35841/neurology-neurorehabilitation.2.1.34-40
Visit for more related articles at Journal of Neurology and Neurorehabilitation ResearchAbstract
The primary subject matter of this case concerns a new approach to neurorehabilitation using trans-lingual neuro-stimulation (TLNS) Technology. Secondary issues examined include the recovery and enhancement of cognitive functioning in a subject with chronic stroke. The case has a difficulty level of four, appropriate for senior level courses who have already covered the topics of neurorehabilitation and neuroplasticity. The case is designed to be taught in one class hour and is expected to require zero hours of outside preparation by students, aside from foundational knowledge of neurorehabilitation techniques and neuroplasticity mechanisms.
Case Description
The primary subject matter of this case concerns a new approach to neurorehabilitation using trans-lingual neuro-stimulation (TLNS) Technology. Secondary issues examined include the recovery and enhancement of cognitive functioning in a subject with chronic stroke. The case has a difficulty level of four, appropriate for senior level courses who have already covered the topics of neurorehabilitation and neuroplasticity. The case is designed to be taught in one class hour and is expected to require zero hours of outside preparation by students, aside from foundational knowledge of neurorehabilitation techniques and neuroplasticity mechanisms.
Case Synopse
Cognitive impairment is a typical consequence of many neurological disorders. It is generally accepted that most improvement for an individual affected by stroke occurs within the first year following the stroke. However, our research shows that it is possible to rehabilitate chronic deficits that result from stroke. TLNS Technology uses a combination of targeted physical exercises and guided meditation with electrotactile stimulation to the tongue with a PoNSTM device. The TCNL completed a 15-month intervention for an 80-year-old woman, 4 years after her stroke. Cognitive improvement was measured using the Stroke Impact Scale (SIS) and Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). The “Memory and Thinking” domain on the SIS demonstrated a 47.3% improvement from baseline. All components of the RBANS improved as a result of the intervention as well, ranging from 10% to 42% improvement from baseline. Recovery demonstrated TLNS stimulation dependence, such that performance across all parameters improved in the first seven months of the intervention, declined during the withdrawal period, and improved again when the intervention was reinstated. We hypothesize that the beneficial effects observed in this case study result from lasting and cumulative neuroplastic changes (functional, synaptic and neuronal) in the brainstem and cerebellum on the cellular and neural network levels, elicited by powerful flow of neural impulses (spikes) from the tongue. This case demonstrates that TLNS balance and gait training can be used to recover and improve cognitive functioning in individuals with chronic stroke. These findings present a new non-invasive brain stimulation technique with applications in cognitive and rehabilitative neurosciences. Additional research is necessary to understand the potential mechanisms of this phenomenon and optimize efficiency of the intervention.
Case Body
Purpose
Trans-lingual neuro-stimulation (TLNS) Technology combines pulsed electrical stimulation of the tongue with speciallydesigned sensory-motor conditioning exercises to effect rehabilitation of balance, posture, and gait symptoms resulting from neurological disorders. The goal of this research was to investigate how neurostimulation can facilitate physiotherapy in the rehabilitation of chronic stroke symptoms. It is important to note that no special cognitive training was applied in this study.
Introduction
Cognitive impairment is a typical consequence of many neurological disorders. It is generally accepted that most improvement for an individual affected by stroke occurs within the first year following the stroke. However, our research shows that it is possible to rehabilitate stroke symptoms from the chronic stage of stroke. TLNS Technology, generally used to rehabilitate balance, posture, and gait, has a surprising impact on the recovery of cognitive function. In a case of chronic stroke, cognitive improvement was an exciting secondary result of a routine procedure designed to rehabilitate balance and gait, without any additional specific training. TLNS Technology uses a combination of physical exercises and meditation with electrotactile stimulation to the tongue with a PoNSTM device.
“Other existing neurostimulation techniques fall into one of two categories; invasive and non-invasive. Invasive techniques, such as deep brain stimulation (DBS) and vagus nerve stimulation (VNS), present a growing treatment option predominantly for neuropsychiatric disorders [1,2]. However, these techniques are rarely applied in treatments after stroke. Reports of disturbing side effects from DBS and VNS treatment have accumulated over time [3-6]. And treatment effects are limited to the severity of symptoms and time since injury. Due to the cost and invasiveness of DBS and VNS, there have been no human studies in healthy adults. Alternatively, TLNS treatment is cost-effective, non-invasive, and is yet to be limited by symptom severity. On the contrary, patients with more severe the symptoms typically experience more improvement using TLNS [7-9].
Non-invasive brain stimulation techniques have shown similarly promising potential to modulate brain plasticity in humans. In stroke specifically, both sensorimotor and higherorder cognitive impairments, such as aphasia and neglect, have demonstrated varying degrees of recoverability [10]. However, few studies have focused on or produced functional gains in the chronic phase of stroke recovery. Currently, cognitive enhancement effects of TMS are brief-not lasting longer than a few minutes or hours after stimulation [11,12]. Whether or not TMS can produce short-term or even longer-term changes in plasticity for post-stroke recovery remains a question for future investigation [13-15]. Like most brain stimulation techniques, depression is the most widely studied condition with TMS [5]. However, clinical benefits are conclusively marginal in the majority of reports and there is still considerable uncertainty regarding the optimal stimulation parameters [5]. Results from large multisite trials to date are mixed and it is unclear which types of TMS may be beneficial in stroke recovery [5].
tDCS is perhaps one of the oldest and simplest ways of focally stimulating the brain. However, very little is understood about what actually occurs in the brain during tDCS stimulation. tDCS seems to induce inhibition and excitation that can last for minutes to an hour, but whether or not therapeutic changes can endure for weeks or months has yet to be determined5. There are currently no clinically useful applications for tDCS.
The present study uniquely explores the potential of cognitive rehabilitation-and more precisely, enhancement above normal levels-in an individual otherwise deemed maximally recovered according to conventional rehabilitation methods. This case meaningfully contributes to the rapidly growing scientific interest in combined positive effect of simultaneously applied physical training, cognitive-learning paradigms, and non-invasive brain stimulation, [15] otherwise known as multidisciplinary therapy [16].
Background
TLNS Technology aims to alleviate symptoms and facilitate recovery of stroke by enhancing the brain’s ability to functionally compensate for neural tissues damaged or compromised as a result of neurological disorders. Electrotactile stimulation (ETS) system for the human tongue can be used to present either information for sensory substitution applications (e.g. vision, or head orientation in balance control), or for neuromodulation and rehabilitation after neural injury. In the present study we expected to see improvement in balance, posture, and gait as a result of TLNS rehabilitative technique. We did not anticipate cognitive improvement. However, cognitive improvement is not an outlandish result considering our theory of simultaneous whole-brain neuroplasticity.
Tactile sensors in the anterior of the tongue are innervated by two cranial nerves (CN): the lingual branch of the trigeminal nerve (CN-V), and the chorda tympani branch of the facial nerve (CNVII). Additionally, receptor densities are highest at the tip of the tongue, slightly lower at the lateral perimeter, and progressively decline toward the posterior and midline. The electrode used in the present study spans all these regions, leading to substantial differences in the perceived intensity of the tactile sensation as a function of the stimulus location.
The tongue, along with the lip and fingertip, differs from other body sites in their specialization for spatial perception acuity. Previous studies [17-20] have demonstrated that the tongue is even more sensitive than the fingertip by mechanical two-point discrimination thresholds. The tongue is uniquely suited for electrotactile stimulation because in the protected environment of the mouth there is no corneal or protective layer of skin typically found on external body surfaces. Additionally, the cutaneous-sensory receptors are close to the surface of the tongue, and it is continuously bathed with saliva, an active electrolyte. Consequently, the tongue is an attractive candidate for a non-invasive electrotactile brain-machine interface. We have found that the tongue requires only about 3% of the voltage (10 V to 20 V), and far less current (1 mA to 4 mA), than the fingertip to achieve equivalent sensation levels.
Methods
Intervention
The TCNL completed a 15-month, four-phase intervention with an 80-year-old female, four years after her stroke (leftsided, ischemic). She exhibited left-sided hemiparesis, deficient posture, gait, and balance as a result of her stroke. The TLNS intervention used a combination of physical exercises, targeted therapy, and guided meditation with the Portable Neuromodulation Stimulator (PoNSTM) device. Following an initial 2-week in-lab training period (phase I), the subject self-administered TLNS intervention at home for seven months (phase II), with periodic in-lab visits for evaluation and modification. Phase III consisted of a one-month withdrawal period (i.e. no exercise or device use). In Phase IV the subject resumed exercising, training, and device use for an additional six months. The subject was evaluated in-lab before TLNS intervention began, after two-weeks, during in-lab training sessions in phase II, at the end of phase III, and every two months in phase IV. “A detailed description of the physical exercise schedule and applied stimulation protocol for cognitive training was published in the textbook, Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects3, Chapter 44, Cranial Nerve Noninvasive Neuromodulation; New Approach to Neurorehabilitation. Briefly, training consists of four parts: [21] movement control training, [22] balance training, [23] gait training, [24] breathing and awareness training, i.e. BAT. Each component is designed to produce beneficial neuroplastic changes by challenging the subject to the limits of their abilities. Assessment of cognitive functions was performed by a psychiatrist using the stroke impact scale (SIS) and repeatable battery for the assessment of neuropsychological status (RBANS). These were secondary outcomes, intended for observational purposes. No specific cognitive training was performed. Data analysis was performed using a standard statistical package that includes GMP [25] and SPSS [26].
Study Schedule (Table 1 in Appendix)
1. Baseline and initial in-lab training-Phase I (two weeks)
2. At-home treatment-Phase II (7 months)
3. Withdrawal-Phase III (1 month)
4. Resumed treatment-Phase IV (6 months)
Instrumentation
The Portable Neurostimulation Stimulator (PoNSTM) system (ver. 2.0) delivers 19-V pulses to the top surface of the tongue.The biphasic waveform is specifically designed to ensure zero net direct current to minimize the potential for tissue irritation. The system delivers triplets of 0.4 μs to 60 μs-wide pulses at 5 ms intervals (i.e. 200 Hz) every 20 ms (50 Hz) to a 143-electrode array of gold-plated circular electrodes (1.50 mm diam., on 2.34 mm centers). While the voltage and pulse timing to each electrode is programmed in the device and cannot be altered, the subject can adjust the stimulus intensity by manipulating a pair of intensity buttons. At any instant in time, one of the 16 electrodes in each of the 9 sectors on the array is delivering stimulation. The remaining electrodes serve as the current return path to ground.
Results
Repeatable battery for the assessment of neuropsychological status (RBANS).
The RBANS is comprised of six domains: Immediate memory, Visuospatial/constructional, Language, Attention, Delayed memory, and a Total scaled score. Raw scores for each domain are converted to index scores, which range from 0-120, with higher scores indicating better functioning. Refer to the appendix for qualitative description of RBANS index scores 17 [27] examined 181 patients with schizophrenia on the alternate forms (A and B) of the RBANS, same used in the present study, with test-retest intervals ranging from 1-134 days. The authors concluded that retest measurement error for the RBANS was comparable to that of WAIS-3/WMS-3, suggesting that the brevity of the RBANS in comparison to these much longer tests does not result in a marked decrease in test-retest stability [27,28].
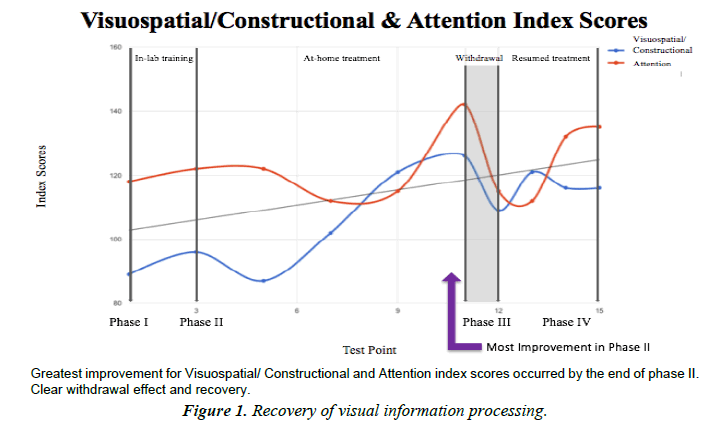
Visuospatial/Constructional
Visuospatial/constructional index is a measure of basic visuospatial perception and the ability to copy a design from a model. Low scores indicate difficulties with processing and using visuospatial information. Visuospatial/constructional category was the most improved index score overall. A marked 42% improvement from baseline to the end of Phase II. The subject's lowest score was within average range, and her best score was superior.
Attention
Attention index is a measure of simple auditory registration and visual scanning and processing speed. Low scores indicate difficulties with basic attention processes and speed of information processing. Attention improvement was not remarkable compared to other categories, although scores did improve overall. Performance was very superior by the end of phase II, dropped to high average after washout, and recovered to very superior by the end of the study (Figure 1).
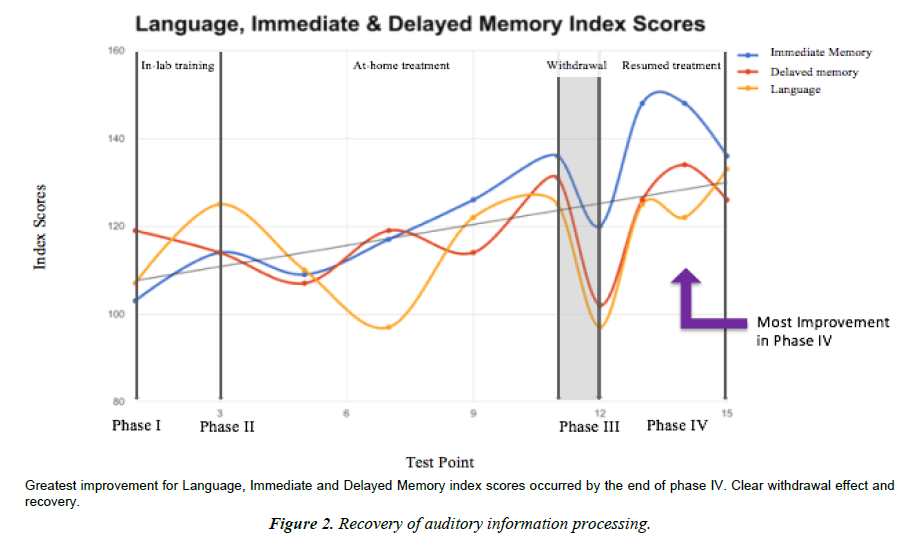
Language
Language index is a measure of expressive language functioning. Low scores indicate difficulties with fluent use of language, including expressive and receptive language. Language index score skyrocketed after washout. Performance at baseline was average and improved to very superior by the end of the study. Interesting that improvement was most marked at the end of the study as opposed to the end of Phase II. Perhaps this is evidence for different learning mechanisms and neuroplastic networks (the type that change quickly, and the type that change slowly or have a latency period).
Immediate memory
The Immediate memory index is a measure of initial encoding and learning of complex and simple verbal information. Low scores indicate difficulties with verbal learning. The immediate memory domain was the least impacted by washout. Performance at baseline was average and increased to very superior by end of study (a 32% increase from start to finish, which is interestingly the same percent increase from baseline to end of Phase II.)
Delayed memory
Delayed Memory index is a measure of delayed recall and recognition for verbal and visual information. Low scores indicate difficulties with recognition and retrieval of information from long-term memory stores. Delayed memory was the least changed category overall, possibly due to a ceiling effect because performance was high average at baseline. Still improved to very superior by the end of the study (Figure 2).
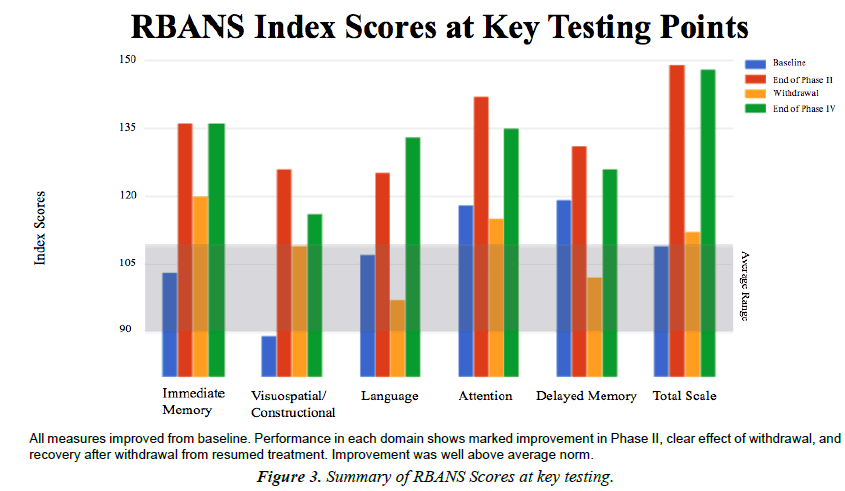
Total scaled score
Dynamics in the total scale score reveal that performance across all subscales was generally low average to average at baseline. The Best scores for half of the subscales occurred at the end of Phase II. Best scores for the other half of the subscales occurred at the end of the study. Performance on all index categories got worse during withdrawal period and recovered when therapy resumed. This suggests a possible dose-dependence effect. It is interesting that half of the index categories improved quickly, while the other half had a latency effect and improved more after withdrawal. Perhaps this alludes to the nature of the neural mechanisms involved with learning and neuroplasticity (Figure 3).
Stroke impact scale (SIS)
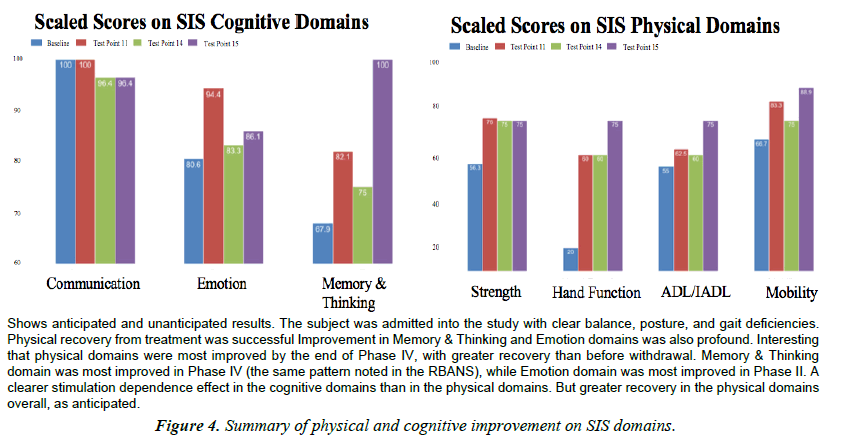
The SIS is a 59-item questionnaire, with higher scores indicated better functioning. Summative scores were generated for each of the 8 domains and normalized to 100. Factor analysis of the SIS 2.0 revealed that the four domains (strength, hand function, mobility, and ADL/IADL) could be summed together to create a physical dimension score (the SIS-16). For the purposes of this case analysis, cognitive domains were similarly grouped together. The SIS was administered a total of four times throughout the study; at baseline, end of Phase II, end of Phase III, and end of Phase IV. Practice effect was a minimal concern because the SIS 2.0 has Adequate to Excellent test-retest reliability in seven out of eight domains [2] (Figure 4).
Physical dimensions
Performance in the Strength domain improved more than one standard deviation above the mean [7], with a minimally clinically important difference (MCID) and nearly minimal detectable change (MDC) [13]. Subject improved 33.21% from baseline to end of study.
Hand Function domain improved nearly two standard deviations greater than the mean [7]. Strong MDC and MCID. Subject improved 275% in this domain by the end of the intervention.
Performance in the ADL/IADL domain was within one standard deviation of the mean [7] from beginning to end of the intervention. There was a MDC and a MCID from baseline to end of study [13]. Scaled scores for the Mobility domain were within one standard deviation of the mean from start to finish. Subject’s performance demonstrated MDC and MCID from baseline to end of study. Improvement on physical subscales was not surprising, as TLNS Technology is designed for balance, posture, and gait rehabilitation [9,10,12,23].
Cognitive dimensions
Improvement in the communication domain was within one standard deviation of the mean. Scaled score at baseline was 100 (perfect) and was maintained overtime. Communication was the least effected domain overall, likely because of a combined ceiling effect and perfect starting point. Improvement in the emotion domain was greater than one standard deviation from the mean for all testing points: greater than two standard deviations above the mean by test point 11 (a 17.1% improvement). Scaled scores for the Memory and Thinking domain improved 20.91% during the first seven months of the intervention (from baseline to the end of Phase II). In the last six months, after a brief withdrawal period, Memory and Thinking continued to improve an additional 21.80%. In total, TLNS intervention improved memory and thinking 47.3% from baseline to the end of the study. Scores reported for the Participation/Role function domain were excluded from the graphic above, but were within one standard deviation of the mean at baseline. Scores improved greater than one standard deviation above the mean by the end of Phase II; decreased during withdrawal; and recovered again to more than one standard deviation above the mean by the end of the intervention. Significant improvement in cognitive subscales occurred without any specialized training.
Discussion
Dynamics in the RBANS total scale score reveal that performance on most subscales at baseline was ‘Low Average’ or ‘Average.’ It is interesting that the best performance on two of the subscales occurred at the end of Phase II, while the best performance for the other three subscales occurred at the end of Phase IV. This may be indicative of a latent learning or latent recovery effect associated with the rehabilitation of certain cognitive functions. What we see is a difference in the rate of improvement of visual information processing and that of auditory (verbal) information processing. These differences may be related to the nature of these independent neural systems. Performance on all index categories declined during the withdrawal period and recovered when therapy resumed, suggesting a stimulation-dependent effect.
Performance on the SIS evidences a similar recovery pattern, in which the Memory and Thinking domain showed profound improvement from baseline to the end of the intervention (the greatest improvement during Phase IV, after withdrawal, just like on the RBANS). The rapid recovery of the emotion domain by the end of Phase II may exemplify the learning curve of cognitive faculties that are susceptible to faster recovery, even years after stroke. Recovery on physical domains was consistent with our hypothesis and other studies using TLNS for the rehabilitation of balance, posture and gait for sufferers of Multiple Sclerosis [23], Parkinson’s, and Traumatic Brain Injury [12]. Recovery and enhancement of cognitive faculties is an inspiring surprise.
A weakness of the SIS is that there is no established minimal detectable change (MDC) or minimally clinically important difference (MCID) for measures of communication, emotion, memory and thinking [29] (refer to Table in the Appendix). Additionally, it is unclear what these scores translate to in daily life [7].
We hypothesize that the beneficial effects observed in this case study result from lasting and cumulative neuroplastic changes (functional, synaptic and neuronal) in the brainstem and cerebellum on the cellular and neural network levels, elicited by powerful flow of neural impulses (spikes) from the tongue [30-32]. There are three possible explanations for the effects seen in this study: [30] Physical exercise improves cognitive functioning, [2] stimulation from the PoNSTM device improves functioning of the cognitive cortexes, and our stimulation improves the speed of sensory processing, whereby holistically improving multiple aspects of functioning. Previous studies of individuals with Multiple Sclerosis, Parkinson’s, and stroke show that TLNS therapy improves multiple facets of cognitive functioning such as multi-tasking, attention, memory, and concentration [9,11]. What’s more is cognitive improvements were transferable to daily life and activities of daily living; something that the majority of other cognitive training clinical studies fail to demonstrate. This particular case is one example of the many promising findings coming out of this novel neurorehabilitative technique.
Fluctuations in performance during active phases of the intervention are likely due to differences in the nature of the internal neural mechanisms. It is a fact that different functional networks have different neural mechanisms. While it makes sense that balance and gait networks have to functionally stay the same from day to day, features of cognitive functioning should be more flexible. Cognitive functioning, as a dynamically assembled network that adapts and responds to its environment and situations, should logically not be a fixed network. Differences between the neural organization and networking of the visual system and auditory system similarly explain why visual processing improved at a faster rate than auditory/verbal processing. First of all, there are a hundred times more input fibers in the visual system than the auditory system. Secondly, the amount of cortex involved in the visual analysis is ten times larger than that for auditory. The place of damage is also a significant consideration, which could impact different systems in different ways.
These results are particularly exciting considering that they expand the scope of symptoms that can be treated using TLNS and showcase some effects that were above and beyond the goals of the initial treatment intervention. The implications of this study will hopefully open the door for future research and treatment for individuals suffering from stroke. It is important to emphasize that this particular patient was cognitively ‘normal’ at the start of the study; scores were average across all RBANS measures for her age and gender. What we show in this case is that TLNS Technology is capable of systematically and continuously improving cognitive functioning above normal levels, in a range of 10% to 42%. Withdrawal in phase III shows a clear drop in cognitive performance, but not below baseline, possibly indicating some retention effect. Recovery of capacity of cognitive functions in phase IV demonstrates that improvement is stimulation dependent. Due to these unparalleled results, we feel obligated to report this data to the scientific community. Furthermore, while much of the existing literature can point to the recovery of cognitive functioning after brain damage, this case provides a rare observation of enhanced cognitive capacity above the norm without initial cognitive impairment.
Conclusion
TLNS Technology combines the use of targeted therapy and non-invasive neurostimulation-delivered directly to the tongueto enhance natural recovery mechanisms. TLNS balance and gait training can be used to recover and improve cognitive functioning in individuals with chronic stroke. In this case, a combination of physical exercise and stimulation dramatically improved cognitive functions even without special cognitive training. These findings present a new non-invasive brain stimulation technique with applications in cognitive and rehabilitative neurosciences. Additional research is necessary to understand the potential mechanisms of this phenomenon and optimize efficiency of the intervention. In conclusion, cognitive deficits associated with chronic stroke may be recoverable. Replication of these findings in future research would be tremendously beneficial for both victims of stroke and the neuroscience community.
References
- Altinay M, Estemalik E, Malone DA. A comprehensive review of the use of deep brain stimulation (DBS) in treatment of psychiatric and headache disorders. Headache: The Journal of Head and Face Pain. 2015;55(2):345-50.
- Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biological psychiatry.2000;47(4):276-86.
- Cyron D. Mental side effects of deep brain stimulation (DBS) for movement disorders: The futility of denial. Frontiers in integrative neuroscience. 2016;10.
- George MS, Aston-Jones G. Noninvasive techniques for probing neurocircuitry and treating illness: Vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). Neuropsychopharmacology. 2010;35(1):301-16.
- Sackeim HA, Rush AJ, GeorgeMS.Vagus nerve stimulation (VNS?) for treatment-resistant depression: Efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25(5): 713-28.
- Helmstaedter C, Hoppe C, Elger CE. Memory alterations during acute high-intensity vagus nerve stimulation. Epilepsy research. 2001;47(1):37-42.
- Tyler ME, Kaczmarek KA, Rust KL, et al. Non-invasive neuromodulation to improve gait in chronic multiple sclerosis: A randomized double blind controlled pilot trial. Journal of neuroengineering and rehabilitation. 2014;11(1):79.
- Wildenberg JC, Tyler ME, Danilov YP, Electrical tongue stimulation normalizes activity within the motion-sensitive brain network in balance-impaired subjects as revealed by group independent component analysis. Brain connectivity. 2011;1(3):255-65.
- Wildenberg JC, Tyler ME, Danilov YP, et al. Altered connectivity of the balance processing network after tongue stimulation in balance-impaired individuals. Brain connectivity.2013;3(1):87-97.
- Schulz R, Gerloff C, Hummel FC. Non-invasive brain stimulation in neurological diseases. Neuropharmacology. 2013;64:579-87.
- Luber B, Lisanby SH. Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS). Neuroimage. 2014;85:961-70.
- Hummel FC, Celnik P, Pascual-Leone A, et al. Controversy: Non-invasive and invasive cortical stimulation show efficacy in treating stroke patients. Brain Stimulation. 2008;14:370e382.
- Zimerman M, Heise KF, Hoppe J, et al. Modulation of training by single-session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke. 43(8):2185-91.
- Yagura H, Miyai I, Seike Y, et al. Benefit of inpatient multidisciplinary rehabilitation up to 1 year after stroke. Archives of physical medicine and rehabilitation. 2003;84(11):1687-91.
- Pleasonton AK. Sensitivity of the tongue to electrical stimulation. Journal of Speech, Language, and Hearing Research. 1970;13(3):635-44.
- Tyler ME, Braun JG, Danilov YP. Spatial mapping of electrotactile sensation threshold and intensity range on the human tongue: Initial results. In Engineering in Medicine and Biology Society, 2009. EMBC 2009. Annual International Conference of the IEEE. 2009;559-562.
- Lozano CA, Kaczmarek KA, Santello M.Electrotactile stimulation on the tongue: Intensity perception, discrimination, and cross-modality estimation. Somatosensory & motor research. 2009;26(2-3):50-63.
- Bach-y-Rita P, Tyler ME, Kaczmarek KA. Seeing with the brain. International journal of human-computer interaction. 2003;15(2):285-95.
- Edwards B, O'connell B. Internal consistency and validity of the stroke impact scale 2.0 (SIS 2.0) and SIS-16 in an Australian sample. Quality of Life Research. 2003;12(8):1127-35.
- Wilk CM, Gold JM, Humber K, et al. Brief cognitive assessment in schizophrenia: Normative data for the Repeatable Battery for the Assessent of Neuropsychological Status. Schizophrenia Research. 2004;70:175-86.
- Randolph, C. Repeatable battery for the assessment of neuropsychological status (RBANS) Supplement 1. Perason Education, Chicago, IL.2008.
- Wildenberg JC, Tyler ME, Danilov YP, et al. Sustained cortical and subcortical neuromodulation induced by electrical tongue stimulation. Brain imaging and behavior. 2010;4(3-4):199-211.
- Wildenberg JC, Tyler ME, Danilov YP, et al. High-resolution fMRI detects neuromodulation of individual brainstem nuclei by electrical tongue stimulation in balance-impaired individuals. Neuroimage. 2011;56(4):2129-37.
- Wildenberg J, Tyler M, Danilov Y, et al. Effects of cranial-nerve non-invasive neuromodulation (CN-NINM) on neural activity as measured by BOLD-FMRI. Proc 17th Scientific Meeting International Society Magn Reson Med. 2009;3316.
- Lin KC, Fu T, Wu CY,et al. "Minimal detectable change and clinically important difference of the stroke impact scale in stroke patients." Neurorehabil Neural Repair. 2010;24(5):486-92.
- Kobeissy FH. (Edn). Brain neurotrauma: molecular, neuropsychological, and rehabilitation aspects. Crc Press 2015.
- Lam T, Noonan VK, Eng JJ. A systematic review of functional ambulation outcome measures in spinal cord injury. Spinal cord. 2008;46(4):246-54.
- Liegl KP, Rust KL, Smith RO. Introduction to the portable neuromodulation stimulator (pons?) device and effects on balance and gait for individuals with traumatic brain injuries.
- Leonard G, Lapierre Y, Chen JK, et al. Noninvasive tongue stimulation combined with intensive cognitive and physical rehabilitation induces neuroplastic changes in patients with multiple sclerosis: A multimodal neuroimaging study. Multiple Sclerosis Journal-Experimental, Translational and Clinical. 2017;3(1):2055217317690561.
- Ziemann U, Siebner HR, Modifying motor learning through gating and homeostatic metaplasticity. Brain Stimulation. 2008;11:60e66.
- Helmstaedter C, Hoppe C, Elger CE. Memory alterations during acute high-intensity vagus nerve stimulation. Epilepsy research. 2001;47(1):37-42.
- Harbourne R, Becker K, Arpin DJ, et al. Improving the motor skill of children with posterior fossa syndrome: A case series. Pediatric Physical Therapy. 2014;26(4):462-8.