Case Report - Otolaryngology Online Journal (2023) Volume 13, Issue 5
COCHLEAR IMPLANT IN A CASE OF PENDRED SYNDROME - BILATERAL SENSORINEURAL HEARING LOSS WITH HYPOTHYROIDISM AND GOITRE
Neha Gupta*, Neeraj Sainy, Sumit Maheshwari, Rohit Mehrotra and Ujjwal MehrotraDepartment of Otolaryngology, Head and Neck Surgery, BMC Hospital, Porto, India
- *Corresponding Author:
- Neha Gupta
Department of Otolaryngology
Head and Neck Surgery
BMC Hospital, Porto, India
E-mail: petermmg@hotmail.com
Received: 21-Aug-2023, Manuscript No. JORL-23-107691; Editor assigned: 23-Aug-2023, Pre QC No. JORL-23-107691(PQ); Reviewed: 06-Sep-2023, QC No. JORL-23-107691; Published: 17-Sep-2023, DOI: 10.35841/2250-0359.13.5.350
Abstract
We present a child of Indian origin with Pendred syndrome who underwent cochlear implant at Mehrotra ENT hospital, Kanpur, India. Patients with Pendred syndrome represent challenging cochlear implant candidate, combining goiter, severe-to-profound hearing loss, and inner ear dysplasias. Cochlear implantation is the proper method for optimal hearing rehabilitation in patients with Pendred syndrome. The genetic background is a mutation of the SLC26A4 gene, coding for a transmembrane protein with anion transport function, called Pendrin. Child was implanted and rehabilitated at our centre. Outcomes in terms of hearing, speech and quality of life are comparable to cases with non-syndromic hearing loss.
Keywords
Pendred Syndrome, Pendrin, Hypothyroidism, Goiter
Introduction
The clinical symptom of severe SNHL or deafness in children has a genetic background in about 65% of the cases, with 85% of them in a non-syndromic appearance [1]. In case of specific accompanying symptoms, the hearing loss is defined as a syndromic form. The most common syndromic form of congenital SNHL or deafness is Pendred syndrome [2]. It is defined as congenital SNHL or deafness and a malfunction of thyroid hormone synthesis. The genetic background is a mutation of the SLC26A4 gene, coding for a transmembraneprotein with anion transport function, called Pendrin [3-5], Pendrin is present in multiple organs, such as the kidney, the airways, the thyroid gland and the inner ear. Besides maintenance of ion composition and pH homeostasis between separated compartments in different organs [5], Pendrin plays a special role during embryological development of the inner ear. However, a mutation of the SLC26A4 gene is not mandatory in case of present SNHL. The distribution of SLC26A4 mutations among patients with SNHL differs among different ethnic populations worldwide. In Asian populations mutations are found with a much higher incidence compared to Caucasian patients [6]. Both malformations, EVA (Enlarge vestibular aqueduct) and MM (mondini’s malformation), are seen as diagnostic hints or radiologic markers for possible Pendred syndrome [7]. Concerning the pathogenesis of hearing loss in patients with proven EVA, EVA seems not to be the causative factor for the hearing loss, but more of an accompanying morphological result of an altered fluid balance in the inner ear during embryological development, due to malfunction of Pendrin.
Case Report
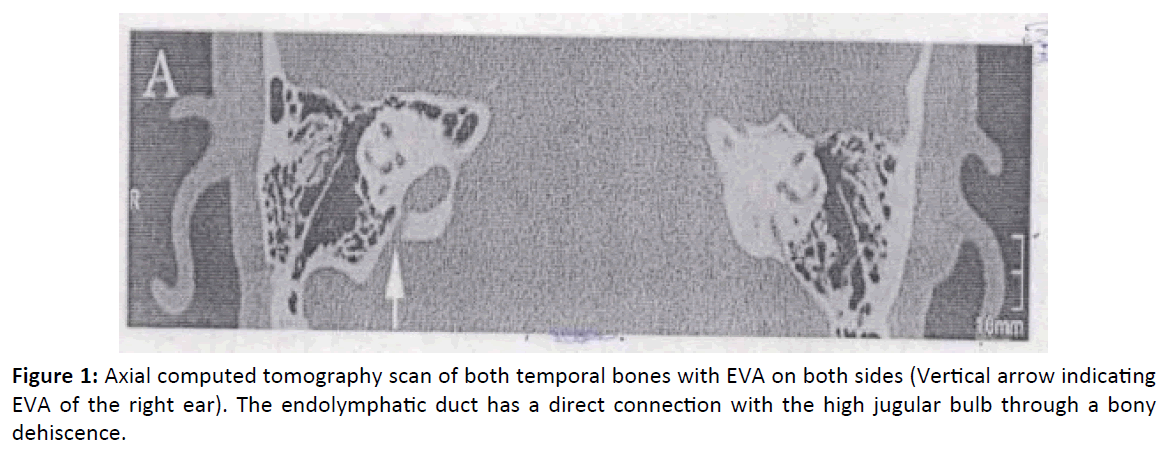
4 year male children presented in our opd at Mehrotra ENT hospital with complain of loss of hearing and speech since birth. Child also presented with diffuse enlarge swelling in central neck suggestive of goiter and episodes of recurrent diarrhea. Child blood evaluation of thyroid hormones confirmed hypothyroid state. A thorough assessment of hearing was done including BERA(brainstem evoked response audiometry), ASSR(auditory steady state response), free field audiometry, impedance and aided audiogram tests following which child was diagnosed with bilateral profound sensorineural hearing loss along with hypothyroidism and goiter - a case of pendred syndrome. Thyroid profile was done. Hypothyroidism was first treated with Eltroxin. Once the thyroid profile (T3, T4, TSH) was normal, the child was planned for cochlear implantation. Radiological investigations which include HRCT (high resolution computed tomography) temporal bone and MRI temporal bone were done for detailed anatomical knowledge. HRCT was abnormal and enlargement of vestibular aqueduct was found. Facial nerves, cochlear nerves, superior and inferior vestibular nerves were normal in course, caliber and signal intensity in bilateral IAC and bilateral prepontine cistern (Figure 1).
On speech and language evaluation, oral peripheral mechanism (lips, teeth, tongue, hard and soft palate, uvula) were normal in appearance as well as function. The voice parameters (pitch, intensity quality), articulation (vowel, consonant) and supra segmental aspects (Intonation, stress/emphasis, pauses, rhythm, rate of speech) were inadequate. Psychological assessment suggestive of verbal IQ (intelligent quotient) in average range and overall IQ in above average range.
His CAP (categories of auditory perception) level before implantation was 0(unaware of environmental sounds). SIR (speech intelligibility rating), score was 1 and GCBI (Glasgow children benefit inventory) poor. Comprehension and expression of ling sound were not very good, discrimination of environmental sounds like bird chirp, door knock were very poor and comprehension and expression of vehicles sounds like horns of traffic were poor too. Child was unable to speak family members name like mama, baba.
After all the routine and specific investigation, Right ear implantation was done. Pericranial flap was elevated, mastoid bone drilled, antrum opened. Posterior tympanostomy done. Round window niche identified and widened, cochlear implant receiver placed in bony skull base and screwed. Round window insertion was done. CSF gusher was present but was managed and controlled. Successful impedance testing done and all electrodes were found working. Flap closed and wound sutured. Digisonic implant was used. Post op was uneventful. X ray skull AP view was done. Electrodes were in cochlea. Switch on was done after 10 days. After 1 year of follow up and regular rehabilitation at our center by our rehabilitation team, child attained CAP level of 5, SIR score of 3 and GCBI index of 51.23 suggestive of moderate benefit. Child achieved 80% comprehension and expression of ling sound. Discrimination of environmental sound achieved 70% and comprehension and expression of vehicles sounds 70%. Child speech of mono and disyllabic words improved by 40%. Comprehension of rhymes improved by 60%.
The auditory performances before and after Cl and the comorbid abnormalities of the patients were analyzed. During their follow-up, the auditory performance analysis scores improved nearly the same as the patients in the same ages without any abnormality (Table 1).
| PRE-IMPLANT STATUS | POST-IMPLANT AND REHABILITATION STATUS | |
|---|---|---|
| CAP | 0 | 5 |
| SIR | 1 | 3 |
| GCBI | Poor | Moderate Benefit |
| Comprehension and Expression of ling sounds | Poor | Achieved 80% |
| Discrimination of environmental sounds like door knock, bird chirps | Very Poor | 30% achieved |
| Comprehension expression of traffic sounds | Poor | 70% achieved |
| Child speech of mono and bisyllabic words | Not able to speak | 40% achieved |
Table 1: Pre Implant Status-Post Implant and Rehabilitation Status.
Discussion
Pendred syndrome is inherited in an autosomal recessive manner, meaning that one would need to inherit an abnormal gene from each parent to develop the condition. This also means that a sibling of a patient with Pendred syndrome has a 25% chance of also having the condition if the parents are unaffected carriers. It has been linked to mutations in the PDS gene, which codes for the pendrin protein (solute carrier family 26, member 4, SLC26A4). The gene is located on the long arm of chromosome (7q31). Mutations in the same gene also cause enlarged vestibular aqueduct syndrome (EVA or EVAS), another congenital cause of deafness [8].
SLC26A4 can be found in the cochlea (part of the inner ear), thyroid and the kidney. In the kidney, it participates in the secretion of bicarbonate. However, Pendred syndrome is not known to lead to kidney problems [9]. It functions as an iodide/ chloride transporter. In the thyroid, this leads to reduced organification of iodine (i.e. its incorporation into thyroid hormone) [10].
People with Pendred syndrome present with a hearing loss either at birth or during childhood the hearing loss is commonly progressive. Thyroid goitre may be present in the first decade. MRI scanning of the inner ear may show widened or large vestibular aqueducts with enlarged endolymphatic sacs and may show abnormalities of the cochlea that are known as Mondini’s dysplasia [11]. Not everyone with Pendred syndrome, however, has an abnormal cochlea. All of those inner ear malformations, including Enlarge vestibular acqueduct and mondini’s malformation, might be a result of a defect within the development of the bony otic capsule. Genetic testing to identify the pendrin gene usually establishes the diagnosis.
Conclusion
Cochlear implantation is the proper method for optimal hearing rehabilitation in patients with Pendred syndrome. Cochlear implant can be an efficient resource for acquiring oral language and reaching complex stages related to hearing abilities, despite the peculiar difficulties caused by the syndrome, it promotes access to sounds, minimizes auditory sensory deprivation, favours interaction with the environment and undeniable quality of life for the child and his family. Although the precise pathogenetic mechanism and the genetic background of deafness in Pendred syndrome have not been completely understood, the preexisting hearing experience represents a positive factor for satisfying hearing outcome. The inner ear malformations if present can cause mild surgical difficulties and extended surgery duration. The cochlear implant surgeon should be aware of these difficulties to avoid complications.
References
- Strutz J (2010) Praxis der HNO-Heilkunde, Kopf- und Halschirurgie: 265 Tabellen. Stuttgart; New York, Thieme.
- Broomfield SJ, Bruce IA, Henderson L, et al. (2013) Cochlear implantation in children with syndromic deafness. Int J Pediatr Otorhinolaryngol 77: 1312-1316.
- Wangemann P (2011) The role of pendrin in the development of the murine inner ear. Cell Physiol Biochem 28: 527-534.
- Ito T, Choi BY, King KA, et al. (2011) SLC26A4 genotypes and phenotypes associated with enlargement of the vestibular aqueduct. Cell Physiol Biochem 28: 545-552.
- Dossena S, Nofziger C, Tamma G, et al. (2011) Molecular and functional characterization of human pendrin and its allelic variants. Cell Physiol Biochem 28: 451-466.
- Huang S, Han D, Yuan Y, et al. (2011) Extremely discrepant mutation spectrum of SLC26A4 between Chinese patients with isolated Mondini deformity and enlarged vestibular aqueduct. J Transl Med 9: 167.
- Griffith AJ, Wangemann P (2011) Hearing loss associated with enlargement of the vestibular aqueduct: mechanistic insights from clinical phenotypes, genotypes, and mouse models. Hear Res 281: 11-17.
- Reardon W, Coffey R, Phelps PD, et al. (1997) Pendred svndrome-100 years of underascertainment?. QJM. 90(7): 443-7.
- Sheffield VC, Kraiem Z, Beck JC, et al. (1996). Pendred syndrome maps to chromosome 7q21-34 and is caused by an intrinsic defect in thyroid iodine organification. Nat Genet 12(4): 424-426.
- Coyle B, Coffey R, Armour JA, et al. (1996) Pendred syndrome (goitre and sensorineural hearing loss) maps to chromosome 7 in the region containing the non-syndromic deafness gene DFNB4. Nat Genet 12(4): 421-423.
- Royaux IE, Wall SM, Kamiski LP, et al. (2001) Pendrin Encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA 98(7): 4221.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref