Research Article - Journal of Clinical Pathology and Laboratory Medicine (2023) Volume 5, Issue 2
Clinicopathological spectrum of intrahepatic cholangiocarcinoma with morphologic subtyping and immunohistochemical analysis of the prognostic biomarkers
Natasha Thomas*
Department of Pathology, Amrita Institute of medical sciences Kochi, Kerala, India.
- Corresponding Author:
- Natasha Thomas
Department of Hematopathology
Erasmus MC-University Medical Center Rotterdam
Netherlands
E-mail: rukiathomas30@gmail.com
Received: 20-Feb-2023, Manuscript No. AACPLM-23-88421; Editor assigned: 22-Feb-2023, PreQC No. AACPLM-23-88421(PQ); Reviewed: 08-Mar-2023, QC No. AACPLM-23-88421; Revised: 11-Mar-2022, Manuscript No. AACPLM-23-88421(R); Published: 18-Feb-2023, DOI:10.35841/aacplm-5.2.137
Citation: Thomas N. Clinicopathological spectrum of intrahepatic cholangiocarcinoma with morphologic subtyping and immunohistochemically analysis of the prognostic biomarkers. J Clin Path Lab Med. 2023;5(2):137
Abstract
Background-Cholangiocarcinoma is the second most common primary liver malignancy second to hepatocellular carcinoma of which intrahepatic cholangiocarcinoma comprises 10%.The disease has a wide morphological spectrum based on which several classification systems have emerged. Also, ICC is usually seen as an incidental finding or there are only non-specific symptoms, due to which diagnosis is often late and the prognosis is consequentially poor. As there are no specific prognostic biomarkers, the aim of this study is to analyse the prognostic significance of IHC based biomarkers, specifically EGFR, MUC1, Fascin and Villin. Method-Prospective and retrospective data of 60 patients were collected where histopathological blocks were available and with a minimum follow up of 6 months. Each case was subjected to treatment with the IHC based markers Fascin, EGFR, MUC1 and Villin and was graded accordingly. The overall survival of each patient was determined and comparison of the IHC markers with the overall survival was determined to assess the prognostic implications of the markers. Results-Statistical analysis showed that there was significance when tumour differentiation, tumour size CEA levels and EGFR expression was compared with survival. Conclusion-Tumour size and differentiation had a significant association with survival with larger tumours with poor differentiation having poor overall survival. Tumours with EGFR expression were mainly poorly differentiated tumours and the overall survival was poor in such cases. Important limitations were that most of the cases were diagnosed on biopsies in which lymph vascular invasion and peri-neural invasion could not be properly ascertained. The procurement of antibodies was also difficult and the grading of IHC findings was not uniform between different studies in existing literature.
Keywords
Cholangiocarcinoma, Prognostic biomarkers, Immuno-histochemical markers.
Introduction
Intrahepatic cholangiocarcinoma arises from the bile ducts proximal to the secondary biliary radicals. It makes up 5-10% of all primary liver malignancies and 10% of all cholangiocarcinoma’s. Most patients are in the 5th to 7th decade and there is slight male preponderance (1.5:1). Patients usually are asymptomatic and even when they are symptomatic, symptoms are non-specific and therefore the diagnosis is often delayed resulting in poor prognosis [1]. The disease is in itself very aggressive and as a result the overall 5 year survival is dismal. It usually arises from a normal liver, but may also be seen in patients with cirrhosis, Hepatitis B and C infections and certain parasites [2]. The disease varies in incidence across different countries with highest in Southeast Asian countries like Thailand [3]. In the past Intrahepatic cholangiocarcinoma was considered to be rare, but in recent years its incidence has been increasing (Figure 1 and 2). Surgical resection with R0 margins is the definitive curative modality. But due to the late presentation of the disease and its aggressive course, surgical resection is usually difficult at the time of diagnosis [4]. CEA and CA19-9 are tumour markers which can be used for diagnosis, but they are nonspecific and hence their usefulness is limited [5].
Proper specific Immunohistochemically markers are imperative for the correct diagnosis of intrahepatic cholangiocarcinoma as it often gets mistaken for hepatocellular carcinoma. Currently, there are no specific IHC markers for the diagnosis of intrahepatic cholangiocarcinoma, and as such further research is required in this area, as specific markers may also help in developing personalized treatment. Currently the studies pertaining to intrahepatic cholangiocarcinoma are being done mainly in the western and south Asian countries and Indian studies are still lacking. And since the incidence is increasing in India, more research needs to be done in this area. So, our study is based on analysis of the prognostic biomarkers EGFR, MUC1, Fascin and Villin and to assess the impact of their expression on overall survival.
Methods
Selection and description of study participants
The research has been approved by the Institutional Review Board. A retrospective and prospective study of 60 patients with histopathology confirmed diagnosis of intrahepatic cholangiocarcinoma from 2014-2022 January, where blocks are retrievable and have a minimum six month follow-up period were included in the study. The Cases are taken with consideration of the history and radiology as well as the histopathological diagnosis. Each case will be subjected to 4 IHC antibodies– EGFR,MUC1,FASCIN and VILLIN and the positivity of each marker and its impact on overall survival will be analysed .The degree of positivity of each IHC will be graded, which is different for each IHC. All cases of perihilar cholangiocarcinoma, distal cholangiocarcinoma and combined HCC-CC were excluded Table 1.
| Tumour Differentiation | Meana | p value | |||
|---|---|---|---|---|---|
| Estimate | Std. Error | 95% Confidence Interval | 0.002 | ||
| Lower Bound | Upper Bound | ||||
| Moderately differentiated | 21.300 | 4.316 | 12.841 | 29.760 | |
| Poorly differentiated | 9.355 | 2.086 | 5.267 | 13.443 | |
| Well differentiated | 53.167 | 11.056 | 31.496 | 74.837 | |
| Overall | 21.130 | 3.498 | 14.274 | 27.986 | |
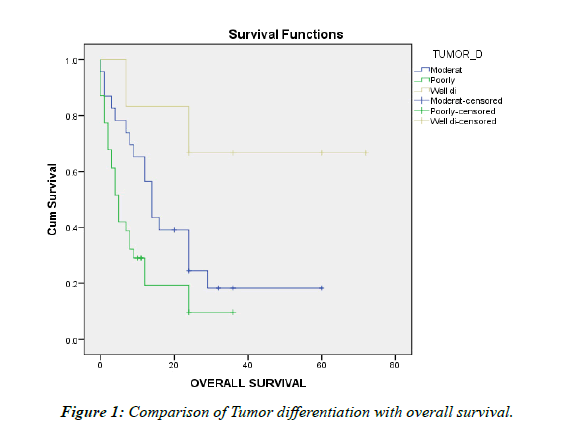
Table 1: Comparison of Tumour differentiation with overall survival
Technical information
The objectives of the study were to determine the prognostic significance of the IHC based biomarkers – Fascin, EGFR, MUC1 and Villin in patients diagnosed as intrahepatic cholangiocarcinoma and to study the clinical spectrum and morphological subtyping in intrahepatic cholangiocarcinoma. The Immunohistochemically markers were graded based on the percentage of cells stained as well as staining intensity.
A grade of 3+ was considered as high expression for each IHC while 1+ and 2+ were considered as low expression.
EGFR
EGFR was evaluated by percentage of stained cells as well as intensity of staining. Only membranous staining was considered: 0-No reactivity/incomplete membranous reactivity in 10% or less of the tumour cells.
1 +- Faint/Barely perceptible incomplete membranous staining in more than 10% of tumour cells.
2 +- Weak to moderate complete membrane staining observed in more than 10% of tumour cells.
3 +- Intense, complete membrane staining in more than 10% of the tumour cells Table 2.
| Tumour Size | Median | p value | |||
|---|---|---|---|---|---|
| Estimate | Std. Error | 95% Confidence Interval | 0.006 | ||
| Lower Bound | Upper Bound | ||||
| ≤5 cm | 24.000 | 5.851 | 12.532 | 35.468 | |
| >5 cm | 7.000 | 1.635 | 3.795 | 10.205 | |
| Overall | 9.000 | 2.046 | 4.991 | 13.009 | |
Table 2: Comparison of Tumour Size with Survival
MUC1
MUC1 was evaluated by percentage of positively stained neoplastic cells. Positivity was based on both luminal membranous and cytoplasmic staining.
0- Less than 5% neoplastic cells.
1. +- More than 5% but less than 20% of tumour cells are positive.
2. +- More than 20% but less than 50% tumour cells are positive.
3. +- More than 50% of tumour cells stained.
FASCIN
FASCIN was evaluated by percentage of positively stained neoplastic cells as well as intensity of staining. Positivity was based on cells having cytoplasmic or membranous staining. Scoring is as follows:
Based on staining intensity
0- No staining.
1-pale yellow staining.
2-buffy staining.
3-strongly brown.
Based on percentage of cells stained
0-No staining.
1-<5% cells stained.
2-25%-50% cells stained.
3-51%-75% cells stained.
4->75% cells stained.
The 2 scores are multiplied and the final score assessed
Negative
1 +- weak positive- score 1-4.
2 +- moderately positive-score 5-8.
3 +- strong positive- score 9-12.
VILLIN
VILLIN was evaluated by intensity and percentage of positively stained cells. Staining is predominantly membranous but cytoplasmic staining also seen. Strong focus on the luminal membrane noted.
0-No cells stained.
1+- mild positive.
2+-moderate positive.
3+-strong positive.
Statistical Analysis
Statistical analysis was performed using IBM SPSS version 20.0 software. Categorical variables were expressed using frequency and percentage. Continuous variables were presented using mean and standard deviation. Kaplan Meier survival analysis was used to study the overall survival and log rank test was used to compare the survival duration between the categorizations of all clinical parameters and tumour features. Univariate Cox regression analysis was done to find the predictors of mortality. A p value of <0.05 was considered to be statistically significant Table 3.
| LVE | Meana | p value | |||
|---|---|---|---|---|---|
| Estimate | Std. Error | 95% Confidence Interval | 0.005 | ||
| Lower Bound | Upper Bound | ||||
| Absent | 64.000 | 7.303 | 49.686 | 78.314 | |
| Present | 15.667 | 3.843 | 8.134 | 23.199 | |
| Overall | 43.231 | 8.350 | 26.864 | 59.598 | |
Table 3: Comparison of Lympho-vascular emboli with Survival (LVE)
Results
60 patients diagnosed as intrahepatic cholangiocarcinoma were taken and were subjected to treatment with 4 IHC -EGFR, MUC1, FASCIN and VILLIN. Statistical significance was noted between Tumor size, Tumor differentiation, AST and CEA levels Lymph vascular emboli and EGFR expression when compared with overall survival Table 4.
| EGFR Expression | Median | p value | |||
|---|---|---|---|---|---|
| Estimate | Std. Error | 95% Confidence Interval | 0.011 | ||
| Lower Bound | Upper Bound | ||||
| Negative | 12.000 | 2.227 | 7.636 | 16.364 | |
| Positive | 1.000 | .943 | 0.000 | 2.848 | |
| Overall | 9.000 | 2.046 | 4.991 | 13.009 | |
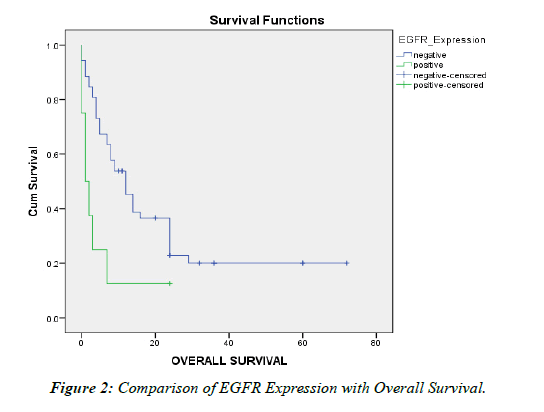
Table 4: Comparison of EGFR Expression with Overall Survival.
Discussion
Primary hepatic malignant epithelial neoplasia includes a variety of tumours, of which Cholangio Carcinoma (CC), is the second most common cancer of the liver after hepato Cellular Carcinoma (HCC)[6]. Intrahepatic cholangiocarcinoma is a very heterogeneous malignancy with respect to histomorphology and molecular perspectives. At present there are no specific biomarkers for prognosticating or for targeted therapy in ICC which warrants a need for more studies.
In our study, 60 histopathological confirmed cases of Intrahepatic cholangiocarcinoma was included, for which blocks were available from the years 2014 through 2022. Most of the patients in our study belonged to ages 50 and above while some of the patients were below 40 years .This was similar to findings from the study conducted by Gupta and Dixon [7]. Other studies conducted by Buettner, et al. Also recorded similar findings (4,8). There was no statistically significant correlation between age and overall survival in our study.
Considering the gender prevalence of the disease, our study comprised of 41 males and 19 females suggesting a male preponderance of the disease which coincided with the findings from studies conducted by Zhang et al, Buettner, et al. Bridgewater, et al.[8,9].Although a study conducted by Razumilava and Gores, indicated that mortality is higher in males with ICC than females , there was no statistically significant correlation between gender and overall survival in our study [10].
The majority of the patients presenting with ICC, who were considered for our study were symptomatic and had advanced disease with poor survival .The symptoms ranged from nonspecific constitutional symptoms like weight loss, malaise and fatigue to more specific ones like abdominal pain. According to Bridgewater et al, in the early stages, the patients are asymptomatic and later, when the disease is advanced, they have non-specific symptoms. This corroborated with the findings from our study. The asymptomatic patients from our study had incidentally detected liver masses when they came to the hospital for other purposes. There was no statistical significance noted on comparing clinical presentation with overall survival. About 14 patients in our study had Chronic liver disease at the time of presentation and 3 cases had a history of Hepatitis B infection, but majority of the patients had a normal background liver. This is similar to the observations made by Bridgewater et al and Razumilava and Gores in their study where they found that most cases of ICC arose from a background of normal liver. Similar to other studies conducted, like studies by Buettner, et al. In our study also, majority of the patients have expired in less than 5 years [11-13].
Lab Parameters
Carbohydrate antigen 19-9 (CA 19-9) is the most commonly used tumour marker for CCA. According to the studies conducted by Blechacz, the accuracy of CA19-9 in distinguishing ICC from HCC is 63% to 67% even though they may be elevated in other malignancies as well (5).Other markers like CEA may also be used but their use is limited due to their non-specificity. In our study, where CA19-9 levels were done, no statistical significance was noted when compared with overall survival. CEA however was found to be a poor prognostic factor with statistical significance which was in accordance with a study by Qiang, et al. [14].
Type of Specimen and the gross characteristics of the lesion
On reviewing existing literature, majority of the studies have included post-surgical resection specimen while in our study we have included both biopsy and hepatectomy specimen, with biopsy specimen being the majority.
Similar to the findings from studies conducted by Nakanuma, et al. and Zhang, et al. our study also showed that the most common gross subtype is the mass forming type. But contrary to their study where the mass forming subtype had poor overall survival, our study did not reveal the same. This may be due to the fact that the patients considered for our study mainly came under the mass forming subtype, while there were only 1 each of the intraductal and mixed subtype .There were no cases with periductal morphology included in our study.
Contrary to the study by He, et al. which documented that majority of the cases involved the left lobe [15],in our study majority of the lesions involved both lobes, followed by right and then the left lobe. In our study, equal numbers of single and multiple lesions were seen as against the study conducted by The et al which documented more number of single lesions than multiple. Comparison of tumor location and tumor number with overall survival did not yield statistical significance. In accordance with the findings documented by Bagante, et al. Where most of the tumors were more than 5 cms [16], in our study also the majority of tumors were more than 5 cms in size and this yielded statistical significance on comparison with overall survival.
Majority of the cases in our study were poorly differentiated tumors and only few were well differentiated .This was contrary to the study by Moon et al were well differentiated tumors were predominant [17] and Shibahara et al were moderately differentiated tumors were predominant[18]. Tumor differentiation was seen to have significant association with overall survival. Distant metastasis, lymph node metastasis, and perineural invasion did not show any statistical significance when compared with overall survival. Lymphovascular Emboli showed statistical significance on comparison with overall survival.
Immuno-histochemical markers
In a meta-analysis conducted by Ruys et al , the markers EGFR,MUC1,MUC4,P27 and Fascin were considered as independent prognostic markers in cholangiocarcinoma [19]. And in a recent study by The et al Villin was found to have a protective effect and SATB 1 was associated with poor prognosis.
In our study, we have used the IHC markers EGFR, Fascin, MUC1 and Villin. In addition, CK7 and CK20 expression was also analysed.
EGFR
EGFR overexpression was noted mainly in poorly and moderately differentiated tumors [20, 21]. In our study, where high expression of EGFR was taken as 3+,this was seen in 6 of poorly ,1 of moderately and 1 of well differentiated cases .This was similar to the findings by Yoshikawa, et al. and yang, et al. The Low expression tumors (grades1+ and 2+) were moderately and well differentiated. EGFR expression showed statistical significance with overall survival implying its association with poor prognosis.
MUC1
According to studies by Moon; and Shibahara, et al. Positive MUC1 expression is associated with poor differentiation, invasion and poor prognosis. In our study, majority of the tumors showing high expression for MUC1 were poorly differentiated and some were moderately differentiated which was in accordance with previous studies. Contrary to the above studies, our study did not show any statistical significance with overall survival.
Fascin
As outlined in the study by Mao et al, high expression of Fascin is associated with tumor dedifferentiation, lymph node metastasis, venous and lymphatic invasion as well as distant metastasis [22]. Also as noted in a study by Iguchi et al, high expression of Fascin was noted in poorly differentiated tumors and within these tumors, larger tumors showed high expression [23]. In our study, high expression was seen in poorly differentiated and moderately differentiated tumors which were in accordance with existing studies. But in our study, no statistical significance was seen with overall survival.
Villin
In the study conducted by The et al ,villin was identified as a favourable prognostic marker, and was lost in poorly differentiated tumors. In our study ,high expression was seen in 3 poorly, 4 moderately and 2 well differentiated tumors. Expression of Villin with overall survival showed borderline significance.
CK7 and CK20
Expression of CK 7 was negatively associated with lymph node metastasis and CK 20 with tumor number .In our study, majority of the tumors with positive CK7 expression showed lymph node metastasis which contradicted the findings outlined by study by He, et al. Both CK7 and C20 did not show statistical significance with survival.
Strengths and limitations of our study
Limitations
Our Sample size was limited due to the infrequency of ICC. Majority of the patients in the study were males .This might have been a cause for the skewed analysis with respect to mortality based on gender. Most of the cases were diagnosed on biopsies, in which Lymphovascular invasion and perineural invasion could not be properly ascertained. The WHO pathologic classification into small and large duct types which shows differences in etiology, molecular signatures and clinical outcome could not be done as most of our diagnosis was from biopsies. Since most of the IHC markers are still used at the research level, procuring them was not easy. The grading used for the IHC markers was not uniform between various studies.
Strengths
Association between tumor features like tumor size and differentiation with survival could be established with statistical significance.Comparison with EGFR and overall survival showed significance which is helpful for targeted therapy.
Conclusion
• Intrahepatic cholangiocarcinoma is mainly seen in the 5th - 8th decade and affected men more than women.
• Many patients were symptomatic but majority had nonspecific symptoms.
• Since most had non-specific symptoms, diagnosis is delayed which leads to poor overall survival.
• CEA and CA19-9 are commonly used to diagnose ICC but they are non-specific. However in our series higher CEA was associated with poorer outcome
• Majority of the tumors developed in the absence of any etiologic factors and in an essentially normal liver.
• Tumor size and differentiation showed statistical significance with overall survival, which indicated that larger tumors and poorly differentiated tumors are associated with poorer overall survival.
• Mass forming type was the most common gross morphology in resection specimen
• Most of the cases were advanced and were poorly differentiated with lymph node and distant metastasis at the time of presentation
• High expression of EGFR, MUC1 and Fascin was seen predominantly in poorly differentiated tumors.
• However EGFR showed statistical significance and villin showed borderline significance with respect to overall survival.
References
- http://www.intechopen.com/books/topics-in-the-surgery-of-the-biliary-tree/intrahepatic-cholangiocarcinoma
- Nakanuma Y, Sato Y, Harada K, et al. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol. 2010;2(12):419.
- Sempoux C, Jibara G, Ward SC, et al. Intrahepatic cholangiocarcinoma: New insights in pathology. 2011; 31(1):49-60.
- Zhang H, Yang T, Wu M, Shen F. Intrahepatic cholangiocarcinoma: Epidemiology, risk factors, diagnosis and surgical management. Cancer Lett. 2016;379(2):198-205.
- Blechacz B. Cholangiocarcinoma: Current knowledge and new developments. Gut and liver. 2017;11(1):13.
- Vijgen S, Terris B, Rubbia-Brandt L. Pathology of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6(1):22.
- Gupta A, Dixon E. Epidemiology and risk factors: Intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6(2):101.
- Buettner S, van Vugt JL, IJzermans JN, et al. Intrahepatic cholangiocarcinoma: Current perspectives. Onco Targets Ther. 2017;10:1131.
- Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268-89.
- Razumilava N, Gores GJ. Cholangiocarcinoma. The Lancet. 2014;383(9935):2168-79.
- Huang L, Xu D, Qian Y, et al. A gene signature is critical for intrahepatic cholangiocarcinoma stem cell self-renewal and chemotherapeutic response. Stem Cell Res Ther. 2022;13(1):292.
- Hassid VJ, Orlando FA, Awad ZT, et al. Genetic and molecular abnormalities in cholangiocarcinogenesis. Anticancer Res. 2009;29(4):1151–6.
- Liau JY, Tsai JH, Yuan RH, et al. Morphological subclassification of intrahepatic cholangiocarcinoma:Etiological, clinicopathological, and molecular features. Mod Pathol. 2014;27(8):1163–73.
- Qiang Z, Zhang W, Jin S, et al. Carcinoembryonic antigen, α-fetoprotein, and Ki67 as biomarkers and prognostic factors in intrahepatic cholangiocarcinoma: A retrospective cohort study. Ann Hepatol. 2021;20:100242.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9071873/
- Bagante F, Spolverato G, Merath K, et al. Intrahepatic cholangiocarcinoma tumor burden: A classification and regression tree model to define prognostic groups after resection. Surgery. 2019;166(6):983–90.
- http://www.spandidos-publications.com/or/22/3/649
- Shibahara H, Tamada S, Higashi M, et al. MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology. 2004;39(1):220–9.
- Ruys AT, Groot Koerkamp B, Wiggers JK, et al. Prognostic biomarkers in patients with resected cholangiocarcinoma: a systematic review and meta-analysis. Ann Surg Oncol. 2014;21(2):487–500.
- Yoshikawa D, Ojima H, Iwasaki M, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98(2):418–25.
- Yang X, Wang W, Wang C, et al. Characterization of EGFR family gene aberrations in cholangiocarcinoma. Oncol Rep. 2014;32(2):700–8.
- Mao X, Chen D, Wu J, et al. Differential expression of FASCIN, e-cadherin and vimentin:Proteins associated with survival of cholangiocarcinoma patients. Am J Med Sci. 2013;346(4):261–8.
- Iguchi T, Aishima S, Taketomi A, et al. Fascin overexpression is involved in carcinogenesis and prognosis of human intrahepatic cholangiocarcinoma: Immunohistochemical and molecular analysis. Hum Pathol. 2009;40(2):174–80.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref