Research Article - Current Pediatric Research (2023) Volume 27, Issue 9
Clinico-epidemiological profile of hepatitis A, B and E virus in paediatric age group patients attending at a tertiary care hospital in India.
Anita Mehta*
Department of Paediatrics, Baba Raghav Das Medical College, Gorakhpur, India
- Corresponding Author:
- Anita Mehta
Department of Pediatrics,
Baba Raghav Das Medical College,
Gorakhpur,
India
E-mail: 25anitamehta@gmail.com
Received: 02-Jun-2023, Manuscript No. AAJCP-23-101031; Editor assigned: 05-Jun-2023, AAJCP-23-101031 (PQ); Reviewed: 20-Jun-2023, QC No. AAJCP-23-101031; Revised: 21-Aug-2023, Manuscript No. AAJCP-23-101031 (R); Published: 29-Aug-2023, DOI: 10.35841/aajcp.27.09.1988-1994
Abstract
Introduction: Viral hepatitis is one of the primary causes of liver illness in children. Acute Viral Hepatitis (AVH) is mostly caused by the Hepatitis A Virus (HAV) and the Hepatitis E Virus (HEV). Hepatitis B, on the other hand, is a major cause of chronic liver disease in children and adolescents. The current investigation sought to determine the seroprevalence and clinical outcomes of HAV, HBV, and HEV in children of different age group.
Materials and methods: During the study period, 142 clinically suspected cases of hepatitis were examined. Data included demographic information; clinical history of illness specifically linked to the hepato-biliary system.
Result: Out of 63 viral hepatitis positive patients, 40 (63.4%) were found HAV positive, 9 (14.2%) were HBV positive, and 14 (22.2%) were HEV positive cases. There were also significant co infection found between hepatitis A and B (n=4, 6.3%) and hepatitis A and E (n=7, 11.1%). Male children (54%) were more prone to all kinds of viral hepatitis than female children (46%), with the most prevalent age group being 0-5 years in HAV and HBV cases and 11-15 years in HEV cases. Icterus (jaundice) was the most prevalent clinical sign in the hepatitis profile, with reporting it, followed by fever hepatomegaly and nausea. In children with hepatitis A and B, ascites, encephalopathy, and acute pancreatitis were the most prevalent consequences.
Conclusion: The most frequent viral hepatitis infection in children is hepatitis A, followed by HEV and HBV. This highlights the importance of routine HAV and HEV testing in AVH patients, particularly for the management of severe infections caused of co-infections condition in other viral hepatitis. Co-infection of HBV in HAV and HEV cases found to be significant in clinical outcome.
Keywords
Acute Viral Hepatitis (AVH), Chronic hepatitis, Co-infection, Hepato-biliary system, Hepatitis A (HAV), Hepatitis B (HBV), Hepatitis E (HEV), Seroprevalence
Introduction
Viral hepatitis in children is a major public health problem worldwide [1]. Hepatitis A Virus (HAV), Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), Hepatitis D Virus (HDV), and hepatitis E virus (HEV) are the major causes of acute and chronic hepatitis and liver infections and are associated with significant morbidity and mortality in developing countries [2].
Although HAV and HEV are the most common cause of acute liver diseases and transmitted by the faeco‑oral route, hepatitis B is the important cause of chronic liver diseases in children and adolescents.
About 1.4 million new cases of HAV infections occur worldwide every year. In the Indian subcontinent, high prevalence rates of HAV cases in the paediatric age group ranged from 31-67%. HAV is a non‑enveloped, 27 nm, heat, and acid and ether‑resistant Ribo-Nucleic Acid (RNA) virus belonging to genus hepatovirus of the family Picornaviridae [3,4].
An estimated 20 million HEV infections occur annually worldwide, leading to 3.3 million symptomatic cases, 44,000 deaths and 3,000 stillbirths. HEV was discovered during an outbreak in New Delhi, India, in 1955 4. HEV is a small non‑enveloped, positive‑sense, single‑stranded RNA virus classified as the single member of the genus ortho-hepetovirus. In developing countries with vast areas of sub-optimal hygiene and sanitary conditions, HAV and HEV infection has assumed endemic proportions. HAV mainly affects children whereas HEV is more common among young adults.
Hepatitis B Virus (HBV) infection was expected to affect 257 million persons globally in 2015, with the Western Pacific and African areas having the largest proportion of positive cases (68% of cases) and North America having the lowest prevalence. In 2017, HBV was responsible for an estimated 29% of all cirrhosis related fatalities globally. HBV is a doublestranded DNA virus of the Hepadnaviridae family that was discovered in 1963 [5,6].
The current trend shows an increase in the incidence of HAV, HBV and HEV infections along with an increase in the rate of HAV HEV co infection and liver disease worldwide. Coinfection with hepatotropic viruses, with prevalence rates ranging from 7 to 24 percent. There has been concern that this co-infection may worsen the condition and give such individuals a poor prognosis, resulting in increased morbidity and death in children. In patients with pre-existing chronic liver disease or co infection with hepatitis B and C, both HAV and HEV infection can worsen the condition. The clinical course of HEV is more problematic than HAV infection, primarily in pregnant females, who contract disease in the second and third trimester. Therefore, HAV-HEV co-infection can lead to serious complications and increased mortality because of acute liver failure in children as well as in adults [7]. Co-infections should be kept in consideration when someone presents with atypical symptoms or unusual disease course, it must be checked for a complete hepatitis profile [8]. There are limited data available in literature defining the pathogenesis and outcome of co-infection with hepatitis A and E, however, other concurrent infections such as hepatitis A with hepatotropic viruses like hepatitis B and C are present in the literature.
In this study, we looked at the seroprevalence and epidemiology of HAV, HBV, and HEV in children of different age groups. The primary objective of this study was to establish the clinical, biochemical, and immunological profile of HAV, HBV, and HEV infection in paediatric age group and secondary objective to determine the incidence of co infection, associated risk factors and clinical outcome of viral hepatitis in a tertiary care hospital.
Materials and Methods
This observational prospective study was conducted in the department of paediatrics and microbiology at a tertiary care hospital in Eastern Uttar Pradesh for a period of one and half years from September 2022 to March 2023. Pre-tested and predesigned questionnaire was used for collecting data. Data included demographic information, risk factors for hepatitis, clinical detail including clinical symptoms and signs, specially related to hepato-biliary system.
The inclusion and exclusion criteria were as follows.
Inclusion criteria
• Patients having age from 2 months to 18 years.
• Patients with symptoms like weakness, lethargy, early
fatigue, joint pains, jaundice (yellow skin) and abdominal
pain; an elevated liver enzyme.
• Patients and their attendants willing to participate.
Exclusion criteria
• Patients with non-viral causes of hepatitis.
• Patients, who Leave Against Medical Advice (LAMA),
could not follow up.
All cases were subjected to the biochemical investigations like Hemoglobin (Hb), Total Leucocyte Count (TLC), Differential Leucocyte Count (DLC), hemoglobin, platelet count, serum bilirubin, Alanine Transaminase (ALT), Aspartate Transaminase (AST), Alkaline Phosphatase (ALP), total proteins, serum albumin, serum globulin, Prothrombin Time (PT), Activated Partial Thromboplastin Time (APTT), blood urea, serum creatinine, blood sugar, serum sodium, serum potassium, Electro Cardiograph (ECG), serum amylase and ultrasonography of the abdomen and thorax. Other specific investigations were also done based on clinical suspicion during the hospital stay or at the time of follow up [9-11]. All children were monitored for the complications during the hospital stay and were discharged once they were a non-febrile for more than 24 hrs, with modest increase in appetite and general well-being.
Serum bilirubin, AST and ALT were done by erba-2 analyser. The serological confirmations of viral hepatitis done for anti HAV immunoglobulin M, HBsAg and anti HEV immunoglobulin M by Enzyme Link Immuno Sorbent Assay (ELISA). For serological study 3-5 ml of blood was collected, and serum was separated and stored at -20°C until further tests were conducted. Tests for anti HAV IgM and anti HEV IgM were done using commercially available kits based on ELISA as per manufacturer’s instruction.
Statistical analysis: Frequencies of various categorical variables were expressed as percentage of total cases. Chi square test, and Fisher’s exact test was used for analysing the qualitative variables using EPI-INFO package. P values less than 0.05 were considered significant.
Results
A total of 142 clinically suspected cases with acute viral hepatitis were tested during the study period, out of which 63 serological confirmed cases were enrolled. Out of 63 viral hepatitis positive cases, 40 (63.4%) HAV, 9 HBV (14.2%) and 14 (22.2%) HEV positive cases were detected. There were also significant co-infection found between hepatitis A and B (n=4, 6.3%) and hepatitis A and E (n=7, 11.1%) (Table 1).
| Serology | Total positive cases n=63 (%) |
|---|---|
| IgM anti‑HAV for Hepatitis A | 40 (63.4%) |
| HBs Ag for Hepatitis B | 9 (14.2%) |
| IgM anti‑HEV for Hepatitis E | 14 (22.2%) |
| Co-infection (Hepatitis A and B) | 04 (6.3%) |
| Co-infection (Hepatitis A and E) | 07 (11.1%) |
Table 1. Detection of serological confirmed acute viral hepatitis cases.
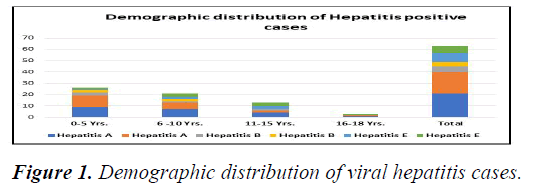
Figure 1 showing age wise distribution of hepatitis A, hepatitis B, hepatitis E and co infection cases. Out of 63 positive viral hepatitis cases 34 (53.9%) male and 29 (46%) were female cases. Hepatitis A (n=21/40, 52.5%) and B (n=5/9, 55.5%) mainly involved less than 5 years old children and hepatitis E (n=6/14, 43%) between 11-15 years. Minimum patients (n=3, 4%) were seen between 16-18 years of age in all types of hepatitis. Over all under 5 is the most common age group involved in all the types of hepatitis and this difference is statistically highly significant p<0.001.
Family history of similar disease was positive in HAV (n=3, 7.5%) and HEV (n=1, 7.1%) cases only. History of similar disease in the community was positive only for HAV (n=4, 10%). According to modified Gopalswamy classification majority of patients of hepatitis A, B and E belongs to class IV, class V and class IV respectively (Table 2).
| Risk factors | Hepatitis A | Hepatitis B | Hepatitis E | |
|---|---|---|---|---|
| N=40 (%) | N=9 (%) | N=14 (%) | ||
| Similar disease in the family | 3 (7.5%) | 0 | 1 (7.1%) | |
| Similar disease in the community | 4 (10%) | 0 | 0 | |
| Source of drinking water | Shallow bore | 2 (5%) | 0 | 0 |
| Deep bore | 12 (30%) | 5 (55.5%) | 7 (50%) | |
| Packed/RO | 6 (15%) | 3 (33.3%) | 4 (11.1%) | |
| Geo distribution of population | Urban | 14 (35%) | 4 (44.4%) | 5 (35.7%) |
| Rural | 26 (65%) | 5 (55.5%) | 9 (64.3%) | |
| Awareness about hepatitis | 2 (5%) | 3 (33.3%) | 0 | |
| Required hospitalization | 18 (45%) | 4 (44.4%) | 07 (50%) | |
Table 2. Risk factors for acute viral hepatitis patients.
Source of drinking water is considered as one of the important risk factors of acute viral hepatitis transmission therefore we included this parameter and split it into three categories; shallow water, deep bore water and packed/RO water consumption. Among HAV, HBV and HEV cases deep bore water was the major source of drinking water reported 30% in HAV, 55.5% in HBV and 50% in HEV cases followed by packed/RO water 15% in HAV, 33% in HBV and 11% in HEV cases and least source of drinking water was shallow water found 5% in HAV.
Geographically distribution of population in viral hepatitis is one of the crucial socio-demographic risk factors. Majority of patients belong to rural area, 40% in HAV, 55% in HBV and 64% in HEV cases. 45% of HAV, 44.4% of HBV and 50% of HEV cases required hospitalization in Pediatric Intensive Care Unit (PICU).
Clinical features of hepatitis A, hepatitis B, hepatitis E and co infection cases were shown in Table 3. The most common clinical feature found in our study was icterus (jaundice), reported in 95% cases of HAV, 88.8% of HBV and 100% cases of HEV and coinfection of hepatitis A and E. Next common feature seen in acute viral hepatitis patients were fever anorexia, nausea/vomiting, splenomegaly and hepatomegaly in varied percentage, shown in Table 3.
| Clinical features | Hepatitis A n=40 (%) | Hepatitis B n=9 (%) | Hepatitis E n=14 (%) | Co-infection (Hepatitis A and E) n=7 (%) | Co-infection HAV and HBV n=4 (%) |
|---|---|---|---|---|---|
| Fever | 28 (70) | 5 (55.5) | 10 (71.4) | 7 (100) | 3 (75) |
| Icterus | 38 (95) | 7 (88.8) | 14 (100) | 7 (100) | 2 (50) |
| Pedal oedema/gen body swelling | 5 (12.5) | 0 | 3 (21.4) | 4 (57.1) | 2 (50) |
| Anorexia | 18 (45) | 7 (77.7) | 11 (78.5) | 5 (71.4) | 4 (100) |
| Pain in abdomen | 20 (50) | 3 (33.3) | 6 (42.8) | 2 (28.5) | 4 (100) |
| Nausea/vomiting | 25 (62.5) | 4 (44.4) | 9 (64.2) | 5 (71.4) | 4 (100) |
| Pruritis | 0 | 0 | 1 (7.1) | 1 (14.2) | 1 (25) |
| GI mucosal/oral bleeding/purpura/petechiae | 2 (5) | 0 | 2 (14.2) | 2 (28.5) | 2 (50) |
| Hepatomegaly | 27 (67.5) | 6 (66.6) | 11 (78.5) | 6 (85.7) | 4 (100) |
| Splenomegaly | 17 (42.5) | 3 (33.3) | 6 (42.8) | 5 (71.4) | 3 (75) |
| Abdominal distension | 12 (30) | 1 (11.1) | 3 (21.4) | 3 (42.8) | 2 (50) |
| Altered sensorium | 8 (20) | 1 (11.1) | 3 (21.4) | 2 (28.5) | 2 (50) |
| Diarrhoea | 11 (27.5) | 2 (22.2) | 4 (28.5) | 3 (42.8) | 1 (25) |
| Pallor | 21 (52.5) | 3 (33.3) | 3 (21.4) | 3 (42.8) | 2 (50) |
Table 3. Clinical features among positive hepatitis patients.
Raised AST, ALT and ALP were the most common findings (76.1%), (69.8%), (62%) respectively seen in hepatitis patients in our study, GGT comparatively less common finding as shown in Table 4. PT was raised in 20% of HAV, 44.4% in HBV and 35.7% cases in HEV. Decreased albumin, hyponatremia, hypokalemia and leukopenia were less common finding. Leukocytosis was seen in 32.5% in HAV, 44.4% in HBV and 7.1% in HEV cases (Table 4).
| Investigations | Hepatitis A n=40 (%) | Hepatitis B n=9 (%) | Hepatitis E n=14 (%) | Total n=63 (%) | |
|---|---|---|---|---|---|
| Elevated AST (>40 U/L) | 33 (82.5) | 6 (66.6) | 9 (64.2) | 48 (76.1) | |
| Elevated ALT (>40 U/L) | 31 (77.5) | 5 (55.5) | 8 (57.1) | 44 (69.8) | |
| Elevated ALP | 27 (67.5) | 4 (44.4) | 8 (57.1) | 39 (62) | |
| Abnormal PT (INR>1.5) | 8 (20) | 4 (44.4) | 5 (35.7) | 17 (27) | |
| Abnormal aPTT (>1.5 times the control) | 3 (7.5) | 2 (22.2) | 3 (21.4) | 8 (12.6) | |
| GGT (<30 IU/L) | 22 (55) | 3 (33.3) | 10 (35.7) | 35 (55.5) | |
| Serum albumin (<3.5 g/dL) | 9 (22.5) | 1 (11.1) | 8 (57.1) | 18 (28.5) | |
| Hyponatremia (<135 MMOL/L) | 5 (12.5) | 2 (22.2) | 3 (21.4) | 10 (16) | |
| Hypokalemia (<3.5 MEQ/L) | 4 (10) | 0 | 5 (35.7) | 9 (14.2) | |
| Serum Urea (>5-18 mg/dL) | 11 (27.5) | 4 (44.4) | 6 (42.8) | 21 (33.3) | |
| Abnormal TLC | <4000 mm3 | 7 (17.5) | 0 | 3 (21.4) | 10 (16) |
| >11000 mm3 | 13 (32.5) | 4 (44.4) | 1 (7.1) | 18 (28.5) | |
Table 4. Biochemical investigations of viral hepatitis cases.
Table 5 showing complications seen in our acute viral hepatitis patients. Encephalopathy was the commonest complication seen in 47 (63.5%) cases, followed by acute pancreatitis, ascites and thrombocytopenia seen in 40 (54%), 38 (51.3%) and 26 (35.1%) cases respectively. In hepatitis A, hepatitis B, hepatitis E and co infection also commonest complications were encephalopathy, acute pancreatitis, ascites and thrombocytopenia. Encephalopathy was seen in 100% cases of HBV and acute pancreatitis was seen in 100% cases of HAV and HEV co infection. Stage 3 encephalopathy was common (44.4%) in HBV patients.
| Complications | Hepatitis A n=40 (%) | Hepatitis B n=9 (%) | Hepatitis E n=14 (%) | Co-infection (HAV and HEV) n=7 (%) | Co-infection (HAV and HBV) n=4 (%) | Total n=74 (%) | |
|---|---|---|---|---|---|---|---|
| Ascites (Sonography) | 15 (37.5) | 6 (66.6) | 9 (64.2) | 5 (71.4) | 3 (75) | 38 (51.3) | |
| Coagulopathy/DIC | 2 (5) | 2 (22.2) | 2 (14.2) | 2 (28.5) | 2 (50) | 10 (13.5) | |
| Encephalopathy | Stage 1 | 2 (5) | 2 (22.2) | 3 (21.4) | 2 (28.5) | 0 | 11 (14.8) |
| Stage 2 | 5 (12.5) | 3 (33.3) | 2 (14.2) | 2 (28.5) | 1 (25) | 14 (19%) | |
| Stage 3 | 11 (27.5) | 4 (44.4) | 3 (21.4) | 2 (28.5) | 3 (75) | 22 (29.7) | |
| Pleural infusion | 3 (7.5) | 0 | 2 (14.2) | 1 (14.2) | 0 | 6 (8.1) | |
| Thrombocytopenia | 16 (40) | 3 (33.3) | 3 (21.4) | 3 (42.8) | 1 (25) | 26 (35.1) | |
| Acute kidney injury | 10 (25) | 2 (22.2) | 3 (21.4) | 3 (42.8) | 1 (25) | 8 (6.7) | |
| Acute pancreatitis | 17 (42.5) | 5 (55.5) | 8 (57.1) | 7 (100) | 3 (75) | 40 (54) | |
| Ac lung injury/ARDS | 1(2.5) | 0 | 1 (7.1) | 1 (14.2) | 0 | 3 (4) | |
| Hypoglycaemia | 2 (5) | 0 | 6 (42.8) | 2 (28.5) | 0 | 10 (13.5) | |
| Shock | 0 | 0 | 1 | 0 | 1 (25) | 2 (2.7) | |
| Blood component therapy | 15 (37.5) | 4 (44.4) | 1 (7.1) | 3 | 2 | 25 (33.7) | |
Note: ARDS (Acute Respiratory Distress Syndrome)
Table 5. Complication and co-infection sequela in viral hepatitis positive cases.
There is no statistically significant difference among three types of hepatitis for the need for mechanical ventilation (p=0.07), duration of ventilation and duration of hospital stay (p=0.05). PICU stay is higher for hepatitis A patients as compared to B and E patients, and this difference is statistically significant (p=0.004). There is no statistically significant difference in mortality among all three types of hepatitis cases.
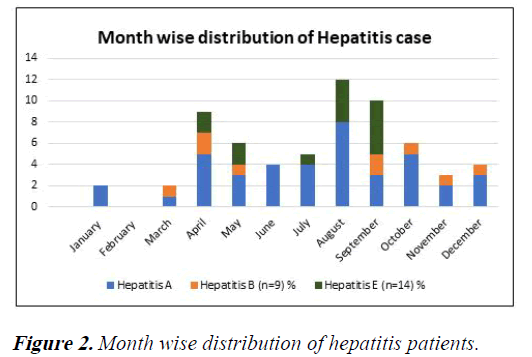
HBV was found around the year, maximum number of HAV and HEV cases was reported during monsoon months (July- September) 37.7% and 71.4% and summer months (April- June) 30% and 28.5% respectively (Figure 2).
Discussion
Viral hepatitis is a serious health concern in paediatrics age group. In the present study, three major hepatitis HAV, HBV and HEV were investigated in paediatrics age group. We found the prevalence of HAV, HBV and HEV 63.44%, 14.2% and 22.2% respectively. HAV and HEV co-infection was 9%. HAV is commonly considers as enterically transmitted etiological agent with high seroprevalence. However, our study also found high HAV and HEV cases prevalence in this region which was accordance with other studies in the Indian subcontinent [12,13]. In our study, the prevalence of HBV positivity was 14%, and co-infection with HAV and HBV was 6%. In Mexico, Melendez et al reported 3.1% HBV and 1.4% of HAV co-infection with HBV [14].
Over all male were more prone to all types of viral hepatitis as compared to female children and most common age group was less than 5 years 52.5% in HAV, 55.5% in HBV cases found positive but surprisingly in HEV cases, 11 to 15 years age group was the most common reported in 42.8% cases. Murlidharan, et al. and Shinde, et al., were also found similar finding [15]. Co‑infection between males and females, there were of no statistical significance difference.
Source of drinking water is regarded as an important risk factor for acute viral hepatitis transmission, we rule out this parameter divided into three categories; shallow water, deep bore and packed/RO water supply in present study. Among HAV, HBV, and HEV cases, deep bore water was the most common source of drinking water, accounting for 30% of HAV cases and 11% of HEV cases and least source of drinking water was shallow water found only 5% in HAV cases. As HAV and HEV infections are transmitted by faeco‑oral route, shortage of drinking water supply in summer and cross‑contamination of drinking water with sewage during monsoons lead to more chances of acquiring these infections. According to studies, the rainy season or polluted water supplies are usually linked with transmission of HAV and HEV cases as a major risk factor.
One of the most important socio-demographic risk factors for viral hepatitis was population distribution. According to Shinde, et al. and Murlidharan, et al., the prevalence of viral hepatitis was greater in rural cases compared to urban ones. These findings are indistinguishable to present study, positivity of viral hepatitis was higher in rural cases reported 40% in HAV, 55% in HBV, 64% in HEV cases.
The commonest clinical features in HAV, HBV and HEV were icterus (jaundice), fever, hepatomegaly and nausea Similarly, Handa S, et al. reported fever, jaundice, vomiting followed by abdominal pain were the most common presenting complaints of the patients. Other studies conducted by Shinde, et al. and Escobedo-Melendez G, et al., showed similar findings, common clinical presentation with jaundice, hepatomegaly (100%), followed by fever and fatigue in HAV, HEV and co infection.
Clinically diagnosed complications were more severe in coinfections of hepatitis A, B and E; ascites, acute pancreatitis and encephalopathy were the predominant clinical complications observed in hepatitis co infection. In coinfection of HAV and HEV, 100% children were diagnosed with acute pancreatitis, 71.4% with ascites and 42.8% with thrombocytopenia. In co infection of HAV and HBV, 75% were diagnosed with ascites and acute pancreatitis, 100% with encephalopathy. Similarly, Palewar, et al., showed that dual infection can lead to severe disease manifestations such as Acute Liver Failure (ALF) and hepatic encephalopathy. Our results were also in accordance with other studies by Samaddar, et al., Mittal et al., and Park, et al.
The major clinical outcome was hospitalization in the PICU, blood component therapy, and mechanical ventilation. Mean length of hospital stay and ventilator was observed average 4 days. Co-infection of HAV, HBV, and HEV cases worsened clinical outcomes, causing acute liver failure, hepatic encephalopathy, and shock. This observation is supported by Mittal, et al. and Park, et al. Furthermore, many patients arrive late at medical facilities due to lack of awareness. This is because early infections do not usually raise bilirubin levels, but they do raise liver enzymes. Therefore, early diagnosis and prompt supportive care through pathogen identification can reduce morbidity and mortality and sequelae.
According to the modified Kuppuswamy classification, the majority of patients in this study who tested positive for HAV, HBV, and HEV were from the lower income, lower-middle, and upper-lower income classes. Murlidharan, et al., reported similar findings. HAV and HEV infections were seen to occur throughout the year, with the highest number of cases recorded in the monsoon months of July to September and the summer months of April to June. Other Indian research has found similar results. HAV and HEV infections are spread through the fecal-oral route, a lack of drinking water supply during the summer, and cross-contamination of drinking water with sewage during the monsoon season, these lead to more chances of acquiring these infections.
Conclusion
Our study revealed that among children, hepatitis A is the most prevalent type of viral hepatitis, followed by HBV and HEV in frequency, the incidence of HAV and HEV co-infection was 9%, and co-infection with HAV and HBV was 5%. This emphasizes routine HAV and HEV testing in acute hepatitis patients, especially for the management of severe infections in HAV HEV co-infections and chronic hepatitis B, high risk groups such as pregnant females, chronic liver disorders, and immunocompromised patients. With a feco oral route of transmission, periodic surveillance, particularly during the summer and monsoon months, is critical for early identification and restriction of outbreaks and epidemics by public health sectors through effective sanitization, cleanliness, and public awareness. Adequate measures are needed to boost sanitation programs and immunization techniques in India in order to control and prevent diseases.
Ethical Consideration
Ethical approval was obtained from ethical committee and written informed consent was obtained from the parents.
Acknowledgement
We are thankful to resident doctors, nurses and technical staff of paediatrics department, microbiology department and RMRC, ICMR India.
References
- Escobedo-Melendez G, Fierro NA, Roman S, et al. Prevalence of hepatitis A, B and C serological markers in children from western Mexico. Ann Hepatol. 2012;11(2):194-201.
- Kayesh MEH, Kohara M, Tsukiyama-Kohara K. Epidemiology and risk factors for acute viral hepatitis in Bangladesh: An overview. Microorganisms. 2022;10(11):2266.
[Crossref] [Google Scholar] [PubMed]
- Samaddar A, Taklikar S, Kale P, et al. Infectious hepatitis: A 3-year retrospective study at a tertiary care hospital in India. Indian J Med Microbiol. 2019;37(2):230-34.
[Crossref] [Google Scholar] [PubMed]
- HandaS, WasimS, KalraBP, RawatA, et al. Clinico-epidemiological profile of hepatitis A virus and hepatitis E virus co-infection in pediatric age group: A hospital based retrospective study. Int J Contemp Pediatr. 2019;6:588-92.
- Castaneda D, Gonzalez AJ, Alomari M, et al. From hepatitis A to E: A critical review of viral hepatitis. World J Gastroenterol. 2021;27(16):1691-1715.
[Crossref] [Google Scholar] [PubMed]
- Kiyasu PK, Caldwell SH. Diagnosis and treatment of the major hepatotropic viruses. Am J Med Sci. 1993;306(4):248-61.
[Crossref] [Google Scholar] [PubMed]
- Naher B, Islam R, Ghosal S, et al. Seroprevalence and co-infection of hepatitis A and hepatitis E viruses in children-A hospital-based study in Bangladesh. J Neonatol Clin Pediatr. 2021;8:080.
- Saeed A, Cheema HA, Assiri A. Hepatitis A and E co-infection with worst outcome. J Coll Physicians Surg Pak. 2016;26(6):31-2.
- Murlidharan S, Sangle AL, Engade M, et al. The clinical profile of children with hepatitis a infection: An observational hospital based study. Cureus. 2022;14(8):28290.
[Crossref] [Google Scholar] [PubMed]
- Wani RT. Socioeconomic status scales-modified Kuppuswamy and Udai Pareekh's scale updated for 2019. J Family Med Prim Care. 2019; 8:1846-9.
[Crossref] [Google Scholar] [PubMed]
- Sarker NR, Saha SK, Ghosh DK, et al. Seropositivity of viral markers in icteric children. Bangladesh Med J. 2014;43(1):26-9.
- Shinde RV, Shinde AR, Patil AD, et al. Co-infection of Hepatitis A and Hepatitis E viruses among the acute viral hepatitis cases in tertiary care hospital –a four years retrospective study. J Pure Appl Microbiol. 2020;14(3):2047-51.
- Palewar MS, Joshi S, Choudhary G, et al. Prevalence of Hepatitis A virus (HAV) and Hepatitis E virus (HEV) in patients presenting with acute viral hepatitis: A 3-year retrospective study at a tertiary care Hospital in Western India. J Family Med Prim Care. 2022;11(6):2437-41.
[Crossref] [Google Scholar] [PubMed]
- Mittal A, Bithu R, Vyas N, et al. Prevalence of hepatitis A virus and hepatitis E virus in the patients presenting with acute viral hepatitis at a tertiary care hospital Jaipur Rajasthan. N Niger J Clin Res. 2016;5:47‑50.
- Kalita D, Paul M, Deka S, et al. Simultaneous infection of Hepatitis A and Hepatitis E viruses amongst acute viral hepatitis patients: A hospital-based study from Uttarakhand. J Family Med Prim Care. 2020;9(12):6130-34.
[Crossref] [Google Scholar] [PubMed]